Footnotes: reference "Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005" (Red Book)
(1) Compounds containing carbon are called organic compounds. You can find out more about the IUPAC conventions for naming organic compounds in the Introduction to Naming Organic Compounds Tutorial.
Inorganic compounds, in general, do not contain carbon but there are notable exceptions such as carbon dioxide (CO2) and carbon monoxide (CO)
Inorganic chemistry and organic chemistry are two separate areas within chemistry because the chemistry of carbon is quite different to the chemistry of other elements.
A binary compound is made up of two (bi) parts, in this tutorial the two parts will be two different non-metals.
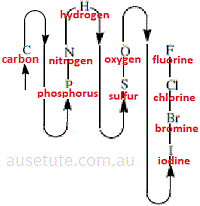
(2) In constructing a stoichiometric name for an inorganic binary non-metallic covalent compound, one element is designated as the electropositive constituent and the other element is designated as the electronegative constituent.
The electropositive constituent is, by convention, the element that occurs first in the sequence given in the diagram.
The order of the elements in the diagram has its basis in consideration of the electronegativity of each element, that is, electronegativity generally decreases going down each group from top to bottom, and increases across a period from left to right.
The same order is used to write molecular formula of binary inorganic molecules, and, to name these molecules.
(3) This eliminates the previous problems of using strict "electronegativity" of elements to order the names of the elements.
Note that compounds containing oxygen are longer named as oxides UNLESS oxygen appears last in the molecular formula based on the diagram.
Previously, compounds containing chlorine and oxygen would have been named as oxides, now they are named as chlorides.
(4) We are using compositional nomenclature to name our binary inorganic compounds, but this is not the only IUPAC system available for nomenclature (and it is not even the most widely used system because it provides only minimal information about the molecule).
There are 3 primary IUPAC systems for naming an inorganic compound:
(a) Compositional nomenclature : requires no understanding of the structure of the connections within the compound.
(Strictly speaking we are using a specific type of compositional nomenclature known as stoichiometric nomenclature because we will be indicating the number of atoms of each element using multiplicative prefixes).
(b) Substitutive nomenclature : requires an understanding of connections within the structure of the compound
(c) Additive nomenclature : requires an understanding of connections within the structure of the compound and this is the most generally applicable system for inorganic compounds
(5) Some names that have been in common useage for a long time are no longer acceptable IUPAC names. Some examples are:
(a) nitric oxide for NO is no longer acceptable. Acceptable names include nitrogen monoxide and nitrogen monooxide.
(b) nitrous oxide for N2O is no longer acceptable. An acceptable IUPAC name is dinitrogen oxide.
(c) phosphine for PH3 is no longer acceptable. The acceptable IUPAC name for this compound as a parent hydride is phosphane.
(6) Herein lies the reason why the "hydrogen names" for some ions are named without a space between "hydrogen" and the name of the rest of the ion.
Consider the compositional names "hydrogen sulfide" and "hydrogen sulfide(2-)".
Hydrogen sulfide could have the formula HS- as well as the formula H2S.
Hydrogen sulfide(2-) could also refer to the formula HS- as well as H2S.
H2S is therefore named dihydrogen sulfide or more commonly hydrogen sulfide, while HS- is named hydrogensulfide, monohydrogensulfide, or hydrogensulfide(1-) (hydrogen names) to avoid confusion.
The "hydrogen name" hydrogensulfide (all one word with no spaces) will ONLY apply to a hydrogen sulfide molecule (H2S) that has lost a proton to become the hydrogensulfide ion, HS-.
(7) More multiplicative prefixes are given in the table below:
| No. atoms |
Prefix |
No. atoms |
Prefix |
No. atoms |
Prefix |
|---|
| 11 |
undeca |
21 |
henicosa |
60 |
hexaconta |
| 12 |
dodeca |
22 |
docosa |
70 |
heptaconta |
| 13 |
trideca |
23 |
tricosa |
80 |
octaconta |
| 14 |
tetradeca |
30 |
triaconta |
90 |
nonaconta |
| 15 |
pentadeca |
31 |
hentriaconta |
100 |
hecta |
| 16 |
hexadeca |
35 |
pentatriaconta |
200 |
dicta |
| 17 |
heptadeac |
40 |
tetraconta |
500 |
pentacta |
| 18 |
octadeca |
48 |
octatetraconta |
1000 |
kilia |
| 19 |
nonadeca |
50 |
pentaconta |
2000 |
dilia |
| 20 |
icosa |
52 |
dopentaconta |
|
|