Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Determining the Products of the Chemical Reaction Between an Acid and a Metal
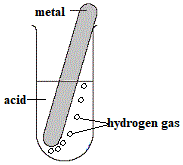 If you place a piece of clean magnesium metal in a test tube with hydrochloric acid, bubbles of gas are given off.
If you place a piece of clean magnesium metal in a test tube with hydrochloric acid, bubbles of gas are given off.
You can use the "pop test" to identify this gas as hydrogen gas.
After the reaction finishes (no more bubbles of gas are produced), you could evaporate off all the liquid and you would be left with a white solid which is called a salt.
You could repeat this chemical reaction using a different acid, sulfuric acid.
In this experiment you add a piece of clean magnesium metal to some sulfuric acid in a test tube.
Bubbles of gas are given off which can also be tested using the "pop test" to identify the gas as hydrogen gas.
You could evaporate off the all liquid and be left with another white solid, another salt.
From these two experiments we could generalise and say that magnesium metal will react with an acid to produce a salt and hydrogen gas.
We can write a word equation to represent this chemical reaction:
| reactants |
→ |
products |
|---|
| magnesium |
+ |
acid |
→ |
salt |
+ |
hydrogen gas |
What if you repeat these experiments using a different metal, zinc for example?
If we add clean zinc metal to hydrochloric acid, bubbles of hydrogen gas are produced, and after evaporation of the liquid, a white salt is left over.
If we add clean zinc metal to sulfuric acid, bubbles of hydrogen gas are produced, and after evaporation of the liquid, a white salt is left over.
From these two experiments we could generalise and say that zinc metal will react with an acid to produce a salt and hydrogen gas.
We can write a word equation to represent this chemical reaction:
| reactants |
→ |
products |
|---|
| zinc |
+ |
acid |
→ |
salt |
+ |
hydrogen gas |
From all of the above experiments, we could make an even more general statement about the products of a chemical reaction between an acid and a metal:
A metal reacts with an acid to produce a salt and hydrogen gas.
and we could write a general word equation to describe this generalisation:
| reactants |
→ |
products |
|---|
| metal |
+ |
acid |
→ |
salt |
+ |
hydrogen gas |
But what is the "salt" made up of? (2)
Naming the Salt Produced in the Reaction Between an Acid and a Metal
The salt produced when a metal reacts with an acid will be made up of 2 parts:
- a metal (the metal used in the experiment)
- a non-metal (from the acid used in the experiment)
The name of the salt is written as two words:
So, in general, the name of the salt produced when a metal reacts with an acid will be either:
- metal chloride (if hydrochloric acid is used)
- metal sulfate (if sulfuric acid is used)
Therefore, we can write some general word equations to describe the chemical reaction between a metal and an acid that produces a salt and hydrogen gas:
| |
reactants |
→ |
products |
|---|
| general word equation: |
metal |
+ |
acid |
→ |
salt |
+ |
hydrogen gas |
|---|
| Example : |
metal |
+ |
hydrochloric acid |
→ |
metal chloride |
+ |
hydrogen gas |
|---|
| Example : |
metal |
+ |
sulfuric acid |
→ |
metal sulfate |
+ |
hydrogen gas |
|---|
De-coding a Word Equation for the Reaction Between an Acid and a Metal
Recall the following facts:
If we are given the following word equation:
iron + hydrochloric acid → iron(2+) chloride + hydrogen
then we can identify the reactants and products of the chemical reaction:
| reactants |
→ |
products |
|---|
| iron + hydrochloric acid |
→ |
iron(2+) chloride + hydrogen |
The reactants are:
The products are:
- iron(2+) chloride (or iron(II) chloride)(3)
- hydrogen
We can also identify the metal and acid reactants, and the salt and gas produced:
| reactants |
→ |
products |
|---|
| metal |
+ |
acid |
→ |
salt |
+ |
hydrogen |
|---|
| iron |
+ |
hydrochloric acid |
→ |
iron(2+) chloride |
+ |
hydrogen |
- metal is iron
- acid is hydrochloric acid
- salt is iron(2+) chloride
- gas is hydrogen
And we can infer what the experimenter may have done: a piece of iron was added to hydrochloric acid.
And we can infer what the experimenter may have observed: bubbles of gas were given off (hydrogen gas).
Footnotes:
(1) More accurately: an active metal reacts with a non-oxidising acid to produce a salt and hydrogen gas.
Active metals include: Group 1 metals (alkali metals), Group 2 metals (alkaline earth metals), aluminium, zinc, titanium, manganese, chromium, iron, cadmium, cobalt, nickel, tin, lead (but not copper, silver, gold nor platinum).
Non-oxidising acids include: inorganic acids like dilute sulfuric acid and hydrochloric acid (but not nitric acid which is an oxidisng acid), and organic acids like acetic acid found in vinegar.
(2) We could perform some tests on the salt to determine what it is made up of:
(i) Flame Test: identifies the metal by the colour of its flame.
(ii) Solubility Tests: identifies the anion by precipitation of an insoluble solid:
(a) Add barium nitrate: if a precipitate forms the salt solution contain sulfate.
(b) If no precipitate forms in (a) then add silver nitrate solution, if a precipitate forms the salt solution contains chloride.
(3) Some metals, like iron, can form ions with different positive charges. Iron can form ions with a charge of 1+, 2+ or 3+.
The name of the cations of iron are therefore: iron(1+), iron(2+), and iron(3+).
The name of the salt formed by these cations must include the name of the metal AND its charge: iron(1+) chloride, iron(2+) chloride, iron(3+) chloride.
Iron can also form covalent compounds.
In this case there is no cation, no positive charge, but we can assign an informal charge based on its oxidation state (which is then referred to as its oxidation state or oxidation number).
When we do this, we use Roman numerals to indicate the oxidation state of the metal.
For iron the oxidation states are iron(I), iron(II), and iron(III).
The Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005 ("Red Book") for the naming of salts (binary inorganic ionic compounds) recommends the use of the charge number (1+, 2+, 3+) instead of the oxidation state (I, II, III).

 If you place a piece of clean magnesium metal in a test tube with hydrochloric acid, bubbles of gas are given off.
If you place a piece of clean magnesium metal in a test tube with hydrochloric acid, bubbles of gas are given off.