UV-Visible Spectroscopy Chemistry Tutorial
Key Concepts
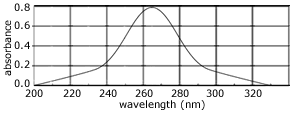
- Some compounds absorb light with wavelengths in the ultraviolet and/or visible range of the electromagnetic spectrum (200 nm to 780 nm).
Valence shell electrons, either bonding or non-bonding electrons, most easily absorb this energy.
The electron in the ground state (lower energy level) absorbs this energy and moves to a higher energy level (excited state).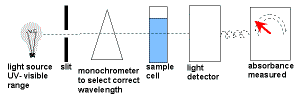
- UV-Visible spectroscopy uses a UV-visible spectrometer.
White light enters a monochromator which narrows down the wavelength band of the radiation.
This monochromatic light is split into two beams.
One beam, the reference beam, passes directly to the detector to measure its intensity.
The other beam passes through the sample which absorbs some of the light before it enters the detector where its intensity is measured.
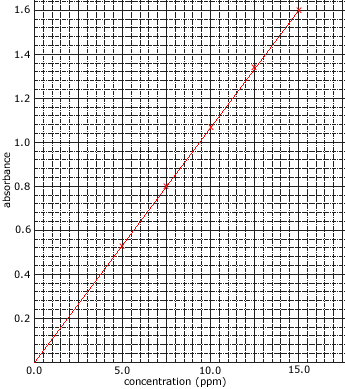
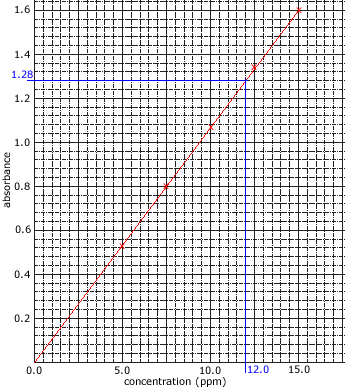
The intensity of the reference beam is compared to the intensity of the other beam at each wavelength in order to give a measurement of the sample's absorption. - The amount of light absorbed is proportional to the concentration of the compound in solution.
Concentrations are often expressed as mg L-1 or ppm. - The amount of light absorbed by the sample is compared to the amount of light absorbed by a set of standards of known concentration.