Acid-Base Direct Titration Calculations Tutorial
Key Concepts
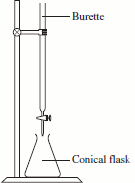
- An acid-base titration is used to determine the concentration of an acid or of a base.
- An acid-base titration involves the progressive addition of one reactant from a burette (buret), often the acid, to a known volume of the other reactant in a conical (erlenmeyer) flask, often the base.
- An Arrhenius acid is a species that dissociates in water to produce hydrogen ions or protons, H+(aq).
An Arrhenius base is a species that dissociates in water to produce hydroxide ions, OH-(aq) - An Arrhenius acid reacts with an Arrhenius base to produce a salt and water in a neutralisation (neutralization) reaction 1:
General word
equation :Arrhenius
acid+ Arrhenius
base→ salt + water General chemical
equation :H-X + M-OH → M-X + H-OH HX + MOH → MX + H2O Net ionic
equation :H+(aq) + OH-(aq) → H2O(l) - The equivalence point of the neutralisation titration is the point at which the moles of H+ is equal to the moles of OH-.
An indicator is used to indicate the equivalence point during a titration by changing colour2.
- The titration experiment is usually conducted several times carefully and the volume of solution used from the burette (buret) recorded (known as a titre).
The volume of solution used in calculations is then the average of all these titres.
- In order to calculate the concentration of an acid, c(acid), we need to know accurately:
(i) the concentration of the base used, c(base), usually in mol L-1
(ii) the volume of the base used, V(base), usually in mL which we convert to L
(iii) the volume of the acid used to neutralise the base, V(acid), usually in mL which we convert to L - In order to calculate the concentration of a base, c(base), we need to know accurately:
(i) the concentration of the acid used, c(acid), usually in mol L-1
(ii) the volume of the acid used, V(acid), usually in mL which we convert to L
(iii) the volume of the base used to neutralise the acid, V(base), usually in mL which we convert to L
