Structure of Disaccharides
Sucrose, lactose and maltose are isomers, they have the same chemical formula, C12H22O11, but different structures.
An ether, or glycosidic, link joins 2 monosaccharides to form a disaccharide.
When two monosaccharides react in a condensation reaction the products are a disaccharide and a molecule of water.
Name
(Molecular Formula) |
Formed from |
Structure |
|---|
sucrose
(C12H22O11) |
| glucose |
+ |
fructose |
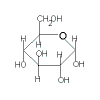 |
+ |
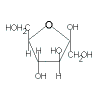 |
|
| → sucrose + H2O |
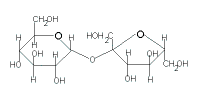 |
|
|
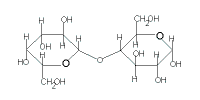
lactose
(C12H22O11) |
| glucose | + | galactose |
 | + | 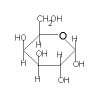 |
|
| → lactose + H2O |
 |
|
|
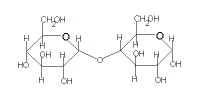
maltose
(C12H22O11) |
| glucose | + | glucose |
 | + |  |
|
| → maltose + H2O |
 |
|






