Saturated and Unsaturated Organic Compounds Chemistry Tutorial
- Saturated compound : an organic molecule in which all the covalent bonds between carbon atoms are single bonds (C−C)
- Unsaturated compound : an organic molecule in which at least one of the covalent bonds between carbon atoms is either a double bond (C=C) or a triple bond (C≡C)
- Hydrocarbon : an organic molecule composed of only carbon atoms (C) and hydrogen atoms (H)
⚛ Saturated hydrocarbon: hydrocarbon molecule in which all the bonds between carbon atoms are single bonds (C−C)
Alkanes are saturated hydrocarbons.
⚛ Unsaturated hydrocarbon: hydrocarbon molecule in which there is at least one :
(i) double bond (C=C), alkenes are unsaturated hydrocarbons
(ii) triple bond (C≡C), alkynes are unsaturated hydrocarbons
- If a compound is known to be a hydrocarbon then we can use bromine water or potassium permanganate solution to test for saturation:
| Tests for Saturation in Hydrocarbons |
|---|
| Type |
Description |
Example |
Tests |
|---|
| Saturated |
ONLY C−C |
alkanes |
Does not decolourise bromine water (stays coloured, yellow-brown). Does not decolourise potassium permanganate solution (stays coloured, pink-purple). |
|---|
| Unsaturated |
either C=C
or C≡C |
alkenes
alkynes |
Bromine water decolourises. Potassium permanganate solution decolourises. |
|---|
| |
For more details of the reaction with bromine water go to halogenation of hydrocarbons.
For more details of the reaction with permanganate go to oxidation of alkenes.
|
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Saturated Hydrocarbons
A hydrocarbon is a compound composed of only carbon atoms (C) and hydrogen atoms (H).
The bonds between carbon atoms, and between carbon and hydrogen atoms, are all covalent bonds.
Each carbon atom can make 4 covalent bonds.
These bonds can be to hydrogen atoms or to other carbon atoms.
If all the covalent bonds between the carbon atoms in the hydrocarbon molecule are single bonds, C−C, then the hydrocarbon molecule is said to be saturated.
The alkane homologous series (CnH2n+2) provides many examples of saturated hydrocarbons:
| Examples of Saturated Hydrocarbon Compounds (only single bonds between carbon atoms) |
|---|
| Molecular structure |
Molecular formula |
Name |
|---|
|
|
CH4 |
methane |
|
|
C2H6 |
ethane |
| |
H
| |
|
H
| |
|
H
| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
C3H8 |
propane |
| |
H
| |
|
H
| |
|
H
| |
|
H
| |
|
| H− |
C |
− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
C4H10 |
butane |
| |
H
| |
|
H
| |
|
H
| |
|
H
| |
|
H
| |
|
| H− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
C5H12 |
pentane |
Even branched-chain alkanes are saturated because all the covalent bonds between carbon atoms are single bonds:
| Examples of Branched-chain Saturated Hydrocarbons (only single bonds between carbon atoms) |
|---|
| Molecular structure |
Molecular formula |
Name |
|---|
| |
|
|
H
| |
|
|
|
| |
H |
H-C-H |
H |
|
| |
| |
|
| |
|
| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
C4H10 |
2-methylpropane |
| |
|
|
H
| |
|
|
|
|
|
| |
H |
H-C-H |
H |
|
H |
|
| |
| |
|
| |
|
| |
|
| |
|
| H− |
C |
− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
C5H12 |
2-methylbutane |
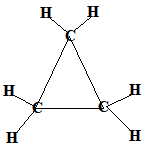
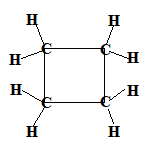
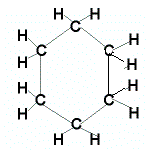
Even if the carbon atoms form a "ring" instead of a "chain", the molecules will still be saturated as long as all the covalent bonds between the carbon atoms are single bonds.
When the carbon atoms all join up to form a ring, we refer to these as cyclic compounds.
We name these cyclic alkanes by placing the prefix "cyclo" in front of the name of the alkane.
A saturated hydrocarbon is an alkane and can be a:
- straight-chain alkane
- branched-chain alkane
- cyclic alkane
Unsaturated Hydrocarbons
An unsaturated hydrocarbon is a molecule made up of only carbon and hydrogen atoms, but 1 or more of the covalent bonds between the carbon atoms is not a single bond, it is either a double bond (C=C) or a triple bond (C≡C). (1)
If the hydrocarbon molecule contains a double bond it is an alkene.
If the hydrocarbon molecule contains a triple bond it is an alkyne.
A molecule belonging to either the alkene or alkyne homologous series is an unsaturated hydrocarbon.
The carbon atoms can be arranged in straight chains, branched chains, or in rings (cyclic compounds), as long as there is at least 1 double bond (C=C) and/or 1 triple bond (C≡C) the molecule will be unsaturated.(2)
| Examples of Unsaturated Organic Compounds (a double bond or triple between carbon atoms) |
|---|
| Homologous Series |
Molecular structure |
Molecular formula |
Name |
|---|
| Alkene |
|
C2H4 |
ethylene
(ethene) |
| H |
|
|
|
H |
|
H |
|
| |
\ |
|
|
| |
|
| |
|
| |
|
C |
= |
C |
− |
C |
−H |
| |
/ |
|
|
|
|
| |
|
| H |
|
|
|
|
|
H |
|
|
C3H6 |
propene
(propylene) |
| H |
|
|
|
H |
|
H |
|
H |
|
| |
\ |
|
|
| |
|
| |
|
| |
|
| |
|
C |
= |
C |
− |
C |
− |
C |
−H |
| |
/ |
|
|
|
|
| |
|
| |
|
| H |
|
|
|
|
|
H |
|
H |
|
|
C4H8 |
but-1-ene
(1-butene) |
| |
|
|
|
H
| |
|
|
|
| H |
|
|
H-C-H |
H |
|
| |
\ |
|
|
| |
|
| |
|
| |
|
C |
= |
C |
− |
C |
−H |
| |
/ |
|
|
|
|
| |
|
| H |
|
|
|
|
|
H |
|
|
C4H8 |
2-methylpropene
(isobutylene) |
| Alkyne |
|
C2H2 |
acetylene
(ethyne) |
|
|
C3H4 |
propyne
(prop-1-yne) |
| |
|
|
H
| |
|
H
| |
|
| H−C≡ |
C |
− |
C |
− |
C |
−H |
| |
|
|
|
H |
|
|
H |
|
|
C4H6 |
but-1-yne
(1-butyne) |
| Cyclic alkene |
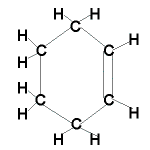 |
C6H10 |
cyclohexene |
An unsaturated hydrocarbon is a compound composed of only carbon and hydrogen atoms, and contains at least one double and/or triple bond between between two of the carbon atoms.
Unsaturated hydrocarbons can be :
- straight-chain alkenes
- branched-chain alkenes
- cyclic alkenes
- straight-chain alkynes
- branched-chain alkynes
Tests for Saturated and Unsaturated Hydrocarbons
Saturated hydrocarbons can be distinguished from unsaturated hydrocarbons in the laboratory because saturated hydrocarbons are less chemically active (reactive) than unsaturated hydrocarbons.
This means alkenes and alkynes more likely to readily react with a chemical reagent than an alkane.
For a qualitative test(3) for unsaturation, a test for the presence or absence or unsaturation, we want to choose a chemical reaction that produces an effect that is easy to observe, a colour change for example.
Two common reagents used to detect whether a hydrocarbon is saturated or unsaturated are:
- bromine water, Br2(aq) : a yellow to brown colour depending on concentration
- potassium permanganate solution, KMnO4(aq) : a pink to purple colour depending on concentration
Bromine Water Test
The preferred test for unsaturation uses bromine water, Br2(aq)(4). Bromine water is an aqueous solution of bromine that is a brown colour if the solution is concentrated, but gradually changes to a more orange colour then to yellow with increasing dilution.
Low-molecular mass hydrocarbons are colourless gases. Gases can be bubbled through the bromine water, if a chemical reaction occurs the colour of the bromine water will "fade", we say the bromine water decolourises.
The decolourisation of the bromine water indicates that the hydrocarbon is unsaturated.
If the bromine water does not decolourise this indicates the presence of a saturated hydrocarbon.(5)
Some hydrocarbons are colourless liquids at room temperture and pressure. The density of these hydrocarbons is less than the density of water so these liquid hydrocarbons float in a layer above the bromine water.
If a few drops of bromine water is added to a saturated liquid hydrocarbon such as cyclohexane and shaken, decolourisation of the bromine water is NOT observed under standard laboratory conditions.
The mixture will separate into 2 distinct layers, a colourless layer of cylcohexane floating on top of the yellow-brown bromine water layer at the bottom.
If a few drops of bromine water is added to an unsaturated liquid hydrocarbon such as cyclohexene and shaken, the bromine water readily decolourises (becomes increasingly less coloured until it is colourless).
If there is an excess of the unsaturated hydrocarbon, then the mixture will settle into 2 distinct but colourless layers with the colourless organic layer floating above the colourless aqueous layer.
Potassium Permanganate Test
Concentrated aqueous solutions of potassium permanganate are a purple colour which fades through to a pink colour as the solution is increasingly diluted.
Low molecular mass gaseous hydrocarbons can be bubbled through potassium permanganate solution.
If the hydrocarbon is saturated there will be no visible colour change (the potassium permanganate solution stays pink-purple).
If the hydrocarbon is unsaturated the potassium permanganate solution will decolourise (pink colour fades to colourless).
For colourless liquid hydrocarbons, add a few drops of potassium permanganate solution and shake.
If the hydrocarbon is saturated there will be no visible colour change (the potassium permanganate solution stays pink-purple).
If the hydrocarbon is unsaturated the potassium permanganate solution will decolourise (pink colour fades to colourless).
Other Saturated and Unsaturated Organic Compounds
The term "saturated" can be applied to any organic molecule that contains only single bonds between the carbon atoms (C−C), even if another functional group is present in the molecule.
So, for example, we can have
- saturated alcohols, alkanes in which 1 or more hydroxyl functional groups (OH) has substituted for a hydrogen atom in the parent alkane.
| |
H
| |
|
OH
| |
H
| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
| |
H
| |
|
H
| |
|
H
| |
|
| HO− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
These compounds are alcohols due to the presence of the OH functional group.
These compounds are saturated because only single bonds are present between carbon atoms (C−C).
- saturated carboxylic acids, alkanes in which a carboxyl functional group (COOH) has substituted for a hydrogen atom in the parent alkane.
| |
H
| |
|
H
| |
O
|| |
|
| H− |
C |
− |
C |
− |
C |
−OH |
| |
|
H |
|
|
H |
|
|
|
|
| |
O
|| |
|
H
| |
|
O
|| |
|
| HO− |
C |
− |
C |
− |
C |
−OH |
| |
|
|
|
H |
|
|
|
|
These compounds are carboxylic acids due to the presence of carboxyl functional groups (COOH).
These compounds are saturated because only single bonds are present between carbon atoms (C−C).
The term "unsaturated" can be applied to any organic molecule that contains a double bond (C=C) or a triple bond (C≡C), even if another functional group is present in the molecule.
So, for example, we can have
- unsaturated alcohols, such as an alkene or alkyne in which 1 or more hydroxyl functional groups (OH) has substituted for a hydrogen atom in the parent unsaturated hydrocarbon.
| H |
|
|
H |
|
H |
|
| |
\ |
|
| |
|
| |
|
| |
C |
= |
C |
− |
C |
−OH |
| |
/ |
|
|
|
| |
|
| H |
|
|
|
|
H |
|
|
|
These compounds are alcohols due to the presence of the OH functional group.
These compounds are unsaturated because the first molecule contains a double bond (C=C) and the second molecule contains a triple bond (C≡C).
- unsaturated carboxylic acids, alkenes or alkynes in which a carboxyl functional group (COOH) has substituted for a hydrogen atom in the parent alkene or alkyne.
| |
H
| |
|
H
| |
O
|| |
|
| H− |
C |
= |
C |
− |
C |
−OH |
| |
|
H |
|
|
|
|
|
|
| |
H
| |
|
H
| |
|
H
| |
|
H
| |
O
|| |
|
| H− |
C |
= |
C |
− |
C |
= |
C |
− |
C |
−OH |
|
These compounds are carboxylic acids due to the presence of carboxyl functional groups (COOH).
These compounds are unsaturated because the first molecule contains one double bond (C=C) and the second molecule contains two double bonds (C=C).
Qualitative tests for saturation become a bit trickier when a functional group, other than a double or triple bond, is present.(6)
Footnotes:
(1) You can have a double bond and a triple bond in the same molecule, these are often referred to as "enynes", or, if there is more than one double and/or triple bond they are called "polyenynes".
(2) This works well for aliphatic hydrocarbons, but ofcourse aromatic compounds are a bit trickier ... refer to the tutorial on benzene for an introduction to this.
(3) A qualitative test for saturation will tell us whether the compound is saturated or unsaturated, but if it is unsaturated then a qualitative test will not tell us how many double and/or triple bonds are present.
A quantitative analysis of an unsaturated compound will tell us how many double and/or triple bonds are present in the compound.
(4) You can also use bromine dissolved in an organic solvent such as tetrachloromethane (carbon tetrachloride) instead of water. Note that both bromine and hydrocarbons are soluble in tetrachloromethane so distinct layers will not form.
When exposed to strong light, alkanes will undergo a substitution reaction with the bromine which will produce hydrogen bromide, HBr, which is only slightly soluble in tetrachloromethane, so you can test the vapour built up above the solution in a test tube with blue litmus paper which will turn red due to the presence of HBr.
The reaction between bromine and an unsaturated hydrocarbon is an addition reaction so no hydrogen bromide (HBr) is produced, so if you test the gas above the solution in the test tube with blue litmus paper then the litmus paper will not change colour.
This bromine solution is not usually used in schools because tetrachloromethane is a known carcinogen.
(5) This is true as long as you don't expose your reaction mixture to strong sunlight or even place it close to bright light bulb!
Exposure to strong light will produce a chemical reaction (a substitution reaction) with saturated hydrocarbons.
(6) Even the presence of conjugation within a molecule alters its stability (and hence reactivity).
A discussion of the possible types of reactions is beyond the scope of this very introductory tutorial.
Be aware that the terms "saturated" and "unsaturated" can be applied to organic compounds other than just hydrocarbons for now.