Protein Structure and Classification Chemistry Tutorial
Key Concepts
Proteins are made up of:
- Carbon (C)
- Hydrogen (H)
- Oxygen (O)
- Nitrogen (N)
- and some proteins contain Sulfur (S)
A protein is a natural polymer, made up of amino acid monomers joined together by peptide bonds (peptide or amide linkages).
- A dipeptide is made up of 2 amino acids joined together by a peptide bond (peptide or amide linkage)
- A tripeptide is made up of 3 amino acids joined together by peptide bonds (peptide or amide linkages)
- A tetrapeptide is made up of 4 amino acids joined together by peptide bonds (peptide or amide linkages)
- A polypeptide is made up of many amino acids joined together by peptide bonds (peptide or amide linkages)
A peptide bond (peptide or amide linkage) is a covalent bond formed between the carbon of the carboxyl group of one amino acid and the nitrogen of the amine group of another amino acid as shown below:
Water is eliminated when the amino acids react to form a protein.
This is known as a condensation reaction, or a condensation polymerisation reaction.
There are four types of protein structure:
- Primary Structure: sequence of amino acids in the chain
- Secondary Structure: the shape of the protein molecule caused by hydrogen-bonding between -C=O and -N-H groups within the chain
- Tertiary Structure: the interaction between R-groups that causes folding and bending
- Quaternary Structure: interactions between protein subunits that result in the protein being classified as fibrous, globular or conjugated.
Denaturation refers to the destruction of the three dimensional structure of a protein that results in the loss of biological activity.
Denaturation of a protein can be caused by:
- a change in pH
- an increase in temperature
- the addition of various chemicals
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Primary Structure
Primary structure of a protein refers to the sequence of amino acids in the chain.
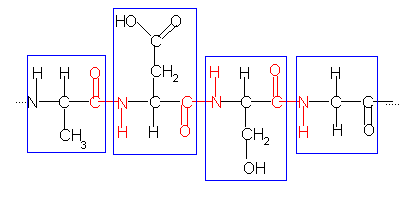
The primary structure of a protein can be shown as:
The blue boxes surround individual amino acids.
The red text shows the position of the peptide bonds (peptide or amide linkages, OC-NH) joining the amino acids together.
Proteins are made up of many amino acids, so a short-hand system has been developed to show the primary structure of proteins.
The following diagram shows part of the primary structure of beef insulin:
| - |
Leu |
- |
Tyr |
- |
Gln |
- |
Leu |
- |
Glu |
- |
Asn |
- |
Cys |
- |
Each 3 letter symbol represents an amino acid, eg, Leu stands for leucine.
Each - represents a peptide bond (peptide or amide linkage) joining the amino acids together.
In 1954 Frederick Sanger was the first to publish a scientific paper on the sequence of a whole protein molecule totalling 51 amino acids for which he was awarded a Nobel Prize.
Secondary Structure
Secondary structure of a protein is the shape of the protein molecule caused by hydrogen-bonding between -C=O and -N-H groups within the chain, the two main shapes are α helix and β sheet.
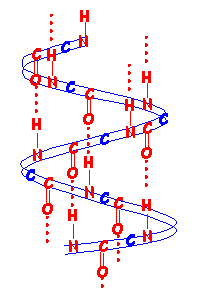
Below is a schematic diagram of an α helix such as is found in wool fibres.
The red dotted lines show the hydrogen bonds between amino acids along the chains maintaining the helical structure.
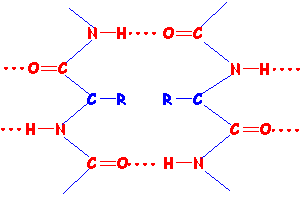
Below is a schematic diagram of a β sheet such as is found in silk.
The red dotted lines show the hydrogen bonds between amino acids along the chains maintaining the sheet structure.
Tertiary Structure
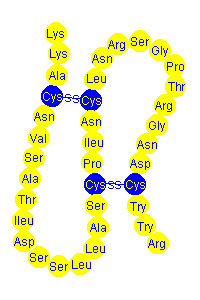
Tertiary structure of a protein is the folding and bending of the protein molecule caused by interaction of the R groups. This interaction may be a result of hydrogen bonding, dipole-dipole interactions, covalent bonding or ionic bonding (salt bridges) depending on the polarity of the R groups. The -SH group in cysteine (Cys) can form disulfide links, -C-S-S-C- , between neighbouring groups in the presence of an oxidant.
Below are two representations of parts of protein molecules showing disulfide bonds (disulfide links) in blue resulting in the molecule folding and bending.
Quaternary Structure
Quaternary structure of a protein are the interactions between protein subunits that result in the protein being classified as fibrous, globular or conjugated, examples of each are shown below.
| Fibrous, or structural (insoluble) |
|---|
| Class |
Comments |
|---|
|
| collagen |
forms connective tissue, comprises 30% of mammalian protein, lacks cysteine & tryptophan, rich in hydroxyproline |
|
| elastins |
forms tendons & arteries |
|
| keratins |
forms hair, quills, hoofs, nails, rich in cysteine & cystine |
|
| Globular (soluble) |
|---|
| Class |
Comments |
|---|
|
| albumins |
eg, egg albumin & serum albumin |
|
| globulins |
eg, serum globulin |
|
| histones |
occur in glandular tissue & with nucleic acids, rich in lysine & arginine |
|
| protamines |
associated with nucleic acids, contain no cysteine, methionine, tyrosine or tryptophan, rich in arginine |
|
| Conjugated (combined with other substances) |
|---|
| Class |
Comments |
|---|
|
| nucleoproteins |
combined with nucleic acids |
|
| mucoproteins |
combined with more than 4% carbohydrates |
|
| glycoproteins |
combined with less than 4% carbohydrates |
|
| lipoproteins |
combined with lipids, such as phosphoglycerides or cholesterol |
| |