Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Synthesis and Naming of Poly(propene) (polypropene)
Polypropene can be produced by the addition polymerisation of the monomer propene, H2C=CH(CH3).
The 2-dimensional structural formula of a molecule of propene is shown below:
Propene has a boiling point of −48°C so it is a gas at room temperature and pressure.
The addition polymerisation of propene results in the formation of a white solid at room temperature and pressure. The structural formula of a short section of the polymer is often represented as shown below:
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
| − |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
Based on the source of this polymer, that is the name of the monomer used to make this polymer, we can name this polymer poly(propene).
The name of the monomer, propene, is enclosed in round brackets and preceded by the prefix "poly".
Poly(propene) is known as a source-based name, that is, the name of the polymer is based on the name of the monomer used to make it.
It is common for the round brackets to be omitted from the name if the resultant name is not ambiguous, so poly(propene) is also known as polypropene.
Notice the repeating unit in this polymer is −H2C−CH(CH3)− (we have highlighted this repeating unit in the diagram below):
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
| − |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
The repeating unit in the structure of this polymer is composed of 2 carbon atoms joined by a single covalent bond, ethane.
There is one branch or side-chain which is a methyl group, CH3. By definition this methyl group is attached to the first carbon of the ethane chain, so we have 1-methylethane.
Since each 1-methylethane unit is joined to another at each end the unit is named as for an alkyl branch with the "yl" suffix and because there are 2 attachment points it is a diyl, the attachments occur at carbons 1 and 2 of the ethane chain hence 1,2-diyl.
The complete name of this polymer based on its structure is therefore poly(1-methylethane-1,2-diyl).
The IUPAC name for this polymer is
- poly(propene) or polypropene (source-based IUPAC name)
- poly(1-methylethane-1,2-diyl) (structure-based IUPAC name)
This polymer is also known as polypropylene. This is not a systematic IUPAC name, it is a traditional name.
The addition polymerisation of propene to produce polypropene requires a catalyst and can be represented by the chemical equation below:
| propene |
catalyst
→ |
polypropene |
| nH2C=CH(CH3) |
catalyst
→ |
-[CH(CH3)−CH2]n- |
Structure of Polypropene
In order to help us understand the structure of polypropene, we will take a closer look at a "polymer molecule" containing just 4 repeating units as shown in the diagram below:
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
H
| |
|
CH3
| |
| H− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
H |
|
The polymer produced by the addition polymerisation of propene is a linear polymer, a single linear chain of monomers.
Notice the fourth carbon atom from the left in the chain, C.
There are four different substituents on this carbon atom:
- H atom
- CH3 group
- CH2-CH(CH3)-CH3 group
- CH2-CH(CH3)-CH2-CH2-CH3 group
This carbon atom has an unsymmetrical arrangement of substituents (2) so the location of these substituents in 3-dimensions will be important to the overall 3-dimensional shape of the polymer.
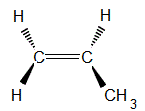
So let's go back and look at the three dimensional structure of propene.
With the C=C in the plane of the page, we have two options for the location of the methyl group; either
(a) coming out of the plane of the page (a solid black wedge in the diagram below)
or
(b) going behind the plane of the page (indicated by broken lines making up the wedge in the diagram below)
| (a) |
|
(b) |
 |
|
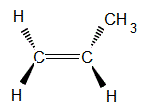 |
Notice that these two structures are identical for a molecule of propene, we can rotate the first molecule around the C=C to make the second structure.
However, when molecules of propene react to form a polymer the orientation of the methyl groups becomes important.
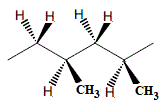
Imagine the polymerisation of propene in which one propene molecule adds to another in a "head-to-tail" reaction to produce a dimer, then another propene molecule adds to this dimer in a "head-to-tail" reaction, and so on.
In a 2-unit section of the polymer chain there are 2 possible positions in 3-dimensions for the methyl groups:
(i) both methyl groups coming out of the plane of the page (which is the same as both methyl groups going behind the page because you can rotate one molecule to make the other)
or
(ii) one methyl group coming out of the plane of the page and the other going behind the plane of the page
| (i) |
|
(ii) |
 |
|
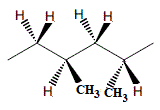 |
If we continue with this ordered arrangement of "head-to-tail" additions of propene to the existing chain we end up with 2 polypropene chains with different arrangements of methyl groups in 3-dimensions.
- If all the methyl groups extend out of the plane of the page we call this isotactic polypropene (3)
- If the methyl groups alternate, the first comes out of the plane, the second behind the plane, and this pattern repeats, then we refer to this as syndiotactic polypropene (4)
As the polymer chain gets longer than 2 repeating units, a third possibility emerges for the location of the methyl groups, that is, instead of an ordered arrangement in which all the methyl groups are behind the plane of the carbon chain or the ordered arrangement in which the methyl groups alternate between behind then in front of the plane, there may be a completely random distribution of the methyl groups along the carbon backbone.
A random arrangement of methyl groups results in a polymer known as atactic polypropene. (5)
Ordered arrangments of methyl groups result in polymer chains that can pack together well, maximising the weak intermolecular forces acting between the polymer chains and resulting in a material that is highly crystalline.
The random distribution of bulky methyl groups in atactic polypropene means that the polymer chains can not pack together neatly, the weak intermolecular forces acting between chains are not maximised, so the resulting material is said to have low crystallinity.
These three types of polypropene are summarised in the table below:
| Type |
Structural formula |
Repeating unit |
Trend in Crystallinity |
|---|
| isotactic polypropene |
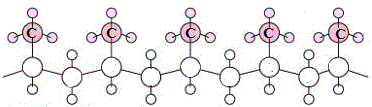 |
|
most crystalline |
|---|
| syndiotactic polypropene |
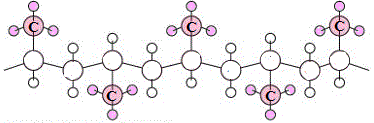 |
| |
H
| |
|
CH3
| |
|
-[ |
C |
−CH2− |
C |
−CH2 |
]-n |
| |
|
CH3 |
|
H |
|
|
|
↓ |
|---|
| atactic polypropene |
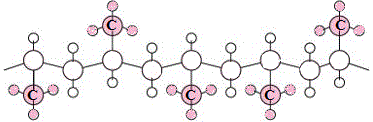 |
none |
least crystalline |
|---|
Properties of Polypropene
Most commercial polypropene is isotactic polypropene which is an opaque material with a melting point(6) between 160 and 166°C.
Isotactic polypropene is essentially linear and highly crystalline.
The hardness, stiffness and high tensile strength of isotactic polypropene is due to its high crystallinity.
Isotactic polypropene is the lightest major plastic having a density of 0.905 g cm-3.
Isotactic polypropene is a poor conductor of electricity, it is an electrical insulator.
Isotactic polypropene is chemically inert and therefore resistant to corrosion.
It does not react with non-oxidising acids and bases, it is resistant to fats and almost all organic solvents except for strong oxidants.
At higher temperatures polypropene can be dissolved in some non-polar solvents.
Polypropene is a linear polymer, that is it does NOT contain cross-links to other polymer chains.
Linear polymers are thermoplastic, that is, they soften with heated.
Because polypropene is a thermoplastic it can be recycled.
After the polypropene is collected for recycling it is cleaned then cut or shredded into small pieces.
These small pieces can be heated and moulded, extruded, or blown into new shapes.
The properties of polypropene can be used to identify an unknown polymer as polypropene:
| Identification of Polypropene |
|---|
| Test |
Result |
|---|
Density
Drop a small piece of polymer into water with a drop of added detergent. |
Floats |
Combustion: Flame Colour
Use tongs to hold a piece of polymer in a flame. |
Burns with a yellow flame with a blue base |
| Combustion: Smell after combustion |
Smells like candle wax |
Scratch Test
Try to scratch the polymer with your fingernail. |
No. (Can't be scratched by a fingernail) |
Thermoplastic
Heat a wire to just below red hot and press it into the polymer. |
Yes. (Hot wire penetrates the polymer) |
Footnotes:
(1) IUPAC is the abbreviation for the International Union of Pure and Applied Chemistry.
IUPAC nomenclature began in 1892 when an international assembly of Chemists met in Geneva, Switzerland, to try to come up with a rational system for naming organic molecules.
The rules for naming organic compounds are still being developed, this page uses the Compendium of Polymer Terminology and Nomenclature IUPAC Recommendations 2008.
The structure-based name is poly(1-methylethane-1,2-diyl).
The traditional name is polypropylene.
The source-based name is poly(propene). An acceptable alternative to this is polypropene because there can be no ambiguity about the structure of the polymer if the round brackets are omitted from the name.
Source-based nomenclature began when we didn't know much about the structure of polymers generally so the name of a polymer was made by adding "poly" to the start of the name of the monomer.
In the last 50 years or so enormous advances have been made in the determination of polymer structures and so the nomenclature of polymers is shifting towards a preference for structure-based names rather than source-based names.
(2) This carbon atom is chiral. The polymer, however, is not optically active because of intermolecular compensation.
(3) "iso" from the Greek isos meaning equal. "Isomers" have the same molecular formula (but different structural formule), "isotopes" have the same atomic number (but different mass numbers), isobars are lines on a map connecting places with the same atmospheric pressure, isotherms are lines on a map connecting places with the same temperature, etc
Formally, an isotactic polymer is a regular polymer composed of tactic macromolecules having essentially only one species of configurational base unit which has chiral or prochiral atoms in the main chain in a unique arrangement with respect to its adjacent constitutional units.
An isotactic polymer consists of meso diads.
(4) Compare "syn" meaning opposed to, the methyl groups are opposed to each other across the C-C bond of the polymer backbone.
Formally, a syndiotactic polymer is a regular polymer composed of tactic macromolecules having alternating enantiomeric configurational base units, which have chiral or prochiral atoms in the main chain in a unique arrangement with respect to their adjacent constitutional units.
A syndiotactic macromolecule consists of racemo diads.
(5) "a" here meaning "not", "tacticity" refers to the orderliness of the repeating units, so an "atactic polymer" is not an ordered arrangement. A random distribution of methyl groups results in a polymer that does not have a regular arrangement of methyl groups in 3 dimensions.
Formally, an atactic polymer is a regular polymer whose molecules have equal numbers of the possible configurational base units in a random distribution.
(6) Perfectly isotactic polypropene has a melting point of 171°C.
Syndiotactic polypropene with 30% crystallinity has a melting point of 130°C.
(7) Note that transparent, more flexible low density poly(ethene) is often used to make the lids for these containers.
(8) HDPE and PET, pol(ethylene terephthalate), are also commonly used to make bottles.
(9) Polymer banknotes are made of a sandwich of layers of polyethene and polypropene.
Read more about polymer banknotes in the March 2014 issue of AUS-e-NEWS.







