Nylon-6,6
Nylon-6,6 was first synthesized by Wallace Carothers in 1934.
Carothers reacted hexandioic acid (also known as adipic acid, a 6-carbon dicarboxylic acid) with 1,6-diaminohexane (also known as hexan-1,6-diamine or hexamethylenediamine, a 6-carbon diamine) in a condensation polymerisation reaction producing the polymer, nylon-6,6, and water. Water is the small molecule eliminated during the condensation reaction.
The first step in this process in which 1 molecule of hexandioic acid reacts with 1 molecule of 1,6-diaminohexane is shown below:
| hexandioic acid | + | 1,6-diaminohexane | → | "a dimer" | + | water |
|
|
+ |
|
270oC
→ |
| O
|| | | O
|| | | H
| | | H
| | |
| HO- | C | -(CH2)4- | C | - | N | -(CH2)6- | N | -H |
|
+ |
H2O |
| (6-carbon dicarboxylic acid) | | (6-carbon diamine) | | | | |
The organic product molecule, "a dimer", has a carboxylic acid functional group at one end, and an amine functional group at the other.
Its carboxylic acid functional group could react with a molecule of 1,6-diaminohexane:
| 1,6-diaminohexane | + | "a dimer" |
|
|
+ |
| O
|| | | O
|| | | H
| | | H
| | |
| HO- | C | -(CH2)4- | C | - | N | -(CH2)6- | N | -H |
|
| ↓ 270oC
| |
| H2O |
+ |
| H
| | | H
| | | O
|| | | O
|| | | H
| | | H
| | |
| H- | N | -(CH2)6- | N | - | C | -(CH2)4- | C | - | N | -(CH2)6- | N | -H |
|
| water | + | "a trimer" |
And, the amine functional group can react with a molecule of hexandioic acid:
| "a trimer" | + | hexandioic acid |
| H
| | | H
| | | O
|| | | O
|| | | H
| | | H
| | |
| H- | N | -(CH2)6- | N | - | C | -(CH2)4- | C | - | N | -(CH2)6- | N | -H |
|
+ |
|
| ↓ 270oC
| |
| | H
| | | H
| | | O
|| | | O
|| | | H
| | | H
| | | O
|| | | O
|| | |
| H2O + | H- | N | -(CH2)6- | N | - | C | -(CH2)4- | C | - | N | -(CH2)6- | N | - | C | -(CH2)4- | C | -OH |
|
| water + "a tetramer" |
And so on, building up the polymer nylon-6,6 (or poly(hexamethylene adipamide) if you prefer).
Nylon-6,10
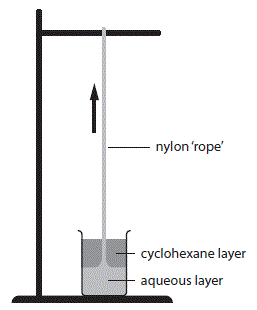 Nylon-6,10 can be prepared in the laboratory by reacting 1,6-diaminohexane,
Nylon-6,10 can be prepared in the laboratory by reacting 1,6-diaminohexane,
H2N(CH2)6NH2, with decanedioyl dichloride, ClOC(CH2)8COCl *.
A solution of decanedioyl dichloride in cylcohexane is floated on an aqueous solution of 1,6-diaminohexane by gently pouring it down the side of the beaker to minimize mixing.
A greyish film of nylon-6,10 forms at the interface and can be pulled out as fast as it is produced forming a long thread (or nylon rope).
For this reason, the demonstration is often referred to as the "nylon rope trick".
The reaction is a condensation polymerisation reaction in which amide links are formed between the monomers and HCl is the small molecule eliminated during the reaction as shown below:
| 1,6-diaminohexane | | decanedioyl dichloride | | nylon-6,10 | | |
| nH2N(CH2)6NH2 |
+ |
nClOC(CH2)8COCl |
→ |
H2N[(CH2)6NHCO(CH2)8]nCOCl |
+ |
(2n-1)HCl |
| 6-carbon diamine | | 10-carbon derivative
of a dicarboxylic acid | | | | |
Nylon-6
Nylon-6 is a polymer that is produced from the monomer caprolactam shown below:
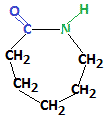
For this reason, nylon-6 is also referred to as polycaprolactam.
First, caprolactam undergoes ring opening with water. The ring opens up between the CO and NH groups:
| caprolactam | | aminocaproic acid monomer |

|
H2O
→
|
| H
| | | | O
|| | |
| H- | N | - | CH2-CH2-CH2-CH2-CH2 | -C | -OH |
|
The aminocaproic acid monomer can then polymerise by forming amide links.
The first step in this polymerisation reaction is shown below:
|
|
+ |
|
250oC
→
|
| H
| | | | O
|| | | H
| | | | O
|| | |
| H- | N | - | (CH2)5 | -C | - | N | - | (CH2)5 | -C | -OH |
|
+ H2O |
The overall polymerization reaction can be represented as:
|
|
250oC
→ |
| H
| | | | O
|| | | H
| | | | O
|| | |
| [- | N | - | (CH2)5 | -C | - | N | - | (CH2)5 | -C | -]n |
|
+ nH2O |
Notice that although water is eliminated during the polymerisation part of the process this synthesis is not generally considered to be a condensation polymerisation reaction, instead it is referred to as a ring-opening polymerisation.
Hydrolysis of Polyamides
The polar amide link in polyamides provides an active site for chemical reaction.
Polyamides can be hydrolysed in strongly acidic conditions. The products of the reaction are a dicarboxylic acid and the salt of an amine.
| polyamide | + | water | + | strong acid | → | dicarboxylic acid | + | amine salt |
| O
|| | | O
|| | | H
| | | H
| | |
| -(- | C | -(CH2)4- | C | - | N | -(CH2)6- | N | -)n- |
|
+ |
(2n-1)H2O |
+ |
2n HCl |
→ |
|
+ |
| H
| | | H
| | |
| n Cl-H- | N+ | -(CH2)6- | N+ | -HCl- |
| |
H | | |
H | |
|
* Note that 1,6-diaminohexane and decanedioyl dichloride are both corrosive and that cylcohexane is highly flammable.
Wearing eye protection and disposable nitrile gloves are essential. Use tweezers to pull out the thread initially.
The room must be well ventilated and there must be no sources of ignition.
Nylon-6,6 can be produced using the rope trick by substituting hexanedioyl dichloride (adipoyl chloride) for decanedioyl dichloride.

 Nylon-6,10 can be prepared in the laboratory by reacting 1,6-diaminohexane,
Nylon-6,10 can be prepared in the laboratory by reacting 1,6-diaminohexane,  COOH
COOH