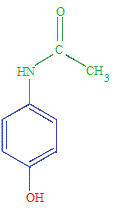
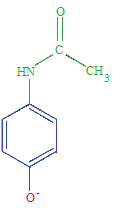
Properties of Paracetamol (acetaminophen)
Aciditity
Paracetamol (acetaminophen) is a weak acid.
The equilibrium position lies very far to the left.
The vast majority of paracetamol molecules in an aqueous solution will be found as the undissociated paracetamol molecules.
At 25oC, paracetamol (acetaminophen) has a 3Ka = 3.09 x 10-10
Solubility
| solvent |
cold water |
hot water |
ethanol |
The difference in the solubility of paracetamol (acetaminophen) in water of different temperatures can be used to separate paracetamol (acetaminophen) from commercially available panadol® or tylenol® as described below. |
|---|
| solubility (g/100 mL) |
1.43 |
5 |
14 |
|---|
| Tablets such as panadol usually contain 500 mg of paracetamol (acetaminophen).
Procedure to determine the paracetamol (acetaminophen) content in commercially available tablets:
- Separation of paracetamol (acetaminophen) from commercially available tablets:
Step 1: Place 2 tablets in 20 mL of propanone (acetone) in a small conical flask suspended in a beaker of warm water.
Step 2: When the tablets have broken up, filter the mixture to remove the undissolved material which will include the binding agents and fillers in the tablets.
Step 3: Leave the filtrate overnight in a fume cupboard to evaporate off the propanone (acetone). The remaining solid is impure paracetamol (acetaminophen).
- Purification of the Paracetamol (acetaminophen)
Step 1: Add 10 mL water to the impure paracetamol (acetaminophen) and heat gently to dissolve it.
Step 2: Filter off any insoluble substances using a hot glass filter funnel
(using a cold funnel will cause the paracetamol to crystallize out in the funnel, reducing the yield)
Step 3: Cool the filtrate to crystallize the paracetamol (acetaminophen).
Step 4: Filter the mixture to separate the insoluble paracetamol (acetaminophen) from the filtrate solution.
Step 5: Dry the pure paracetamol (acetaminophen) in a warm oven.
Step 6: Weigh the dry pure paracetamol.
Step 7: Calculate the mass of paracetamol (acetaminophen) in 1 tablet and compare this to the manufacture's claim on the packet.
|
| Safety Concerns |
|---|
Propanone (acetone) is volatile and highly flammable.
Keep away from flames.
Work in a well-ventilated area (or fume cupboard)
Wear eye protection (safety glasses or goggles).
DO NOT taste the paracetamol!
|
|
It is possible to buy fizzy paracetamol tablets.
In these tablets the paracetamol has been mixed with citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid) and sodium hydrogencarbonate (sodium bicarbonate).
When placed in water, the citric acid and sodium hydrogencarbonate in the tablet react to produce bubbles of carbon dioxide.
The bubbles of carbon dioxide gas help break the tablet up into smaller pieces that are easier to swallow.
Synthesis of Paracetamol (acetaminophen)
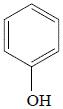
Paracetamol (acetaminophen) can be synthesized from phenol in three steps:
Step 1:nitration of phenol
Phenol (hydroxybenzene) will react with sodium nitrate (an oxidizing agent) in the presence of sulfuric acid to produce a mixture of structural isomers of nitrophenol.
 |
H2SO4
→
NaNO3(aq) |
 |
+ |
 |
phenol
(hydroxybenzene) |
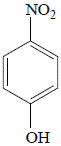
|
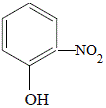
4-nitrophenol
(p-nitrophenol)
25% yield |
|
2-nitrophenol
(o-nitrophenol)
36% yield |
When concentrated sulfuric acid (H2SO4) is added to sodium nitrate (NaNO3) the following reaction occurs:
H2SO4 + 2NaNO3 → Na2SO4 + 2HNO3
Then, in excess sulfuric acid, reactive nitronium ion, NO2+, is produced:
HNO3 + H2SO4 → NO2+ + HSO4- + H2O
The nitronium ion, NO2+, attacks the benzene ring of phenol to produce a mixture of various structural isomers of nitrophenol.
The OH (hydroxyl) functional group of phenol (hydroxybenzene) is said to activate the benzene ring at the 2- and 4- positions. This results in the formation of 2-nitrophenol and 4-nitrophenol.
The 3- and 5- positions of the benzene ring are not activated so 3-nitrophenol and 5-nitrophenol are NOT produced.
4-nitrophenol can be separated from the mixture containing 2-nitrophenol:
- by steam distillation: 2-nitrophenol forms fewer hydrogen bonds with water or other nitrophenol molecules than 4-nitrophenol so it is more volatile in steam than 4-nitrophenol
- or by column chromatography: 2-nitrophenol is less polar than 4-nitrophenol so it has less affinity for silica than 4-nitrophenol.
Step 2: reduction of a nitro group to an amine
In carbon chemistry (organic chemistry) a reduction reaction has occurred if4:
- a molecule loses oxygen
OR
- a molecule gains hydrogen
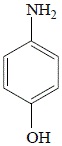
In the reaction shown below, oxygen is lost from the nitro group of 4-nitrophenol and hydrogen is added to form 4-aminophenol, so the reaction is a reduction reaction:
| In the laboratory: |
|
Industrial preparation: |
 |
NaBH4
→
Pd/1 M NaOH |
 |
|
 |
H2
→
Pt catalyst |
 |
| 4-nitrophenol |
|
4-aminophenol
74% yield |
|
4-nitrophenol |
|
4-aminophenol
|
A catalyst such as palladium in the laboratory reaction, or platinum in the industrial reaction, is required to provide a surface for the reaction to take place on.
The 4-nitrophenol molecules are held to the surface of the catalyst by weak forces of attraction, which then weakens the strong covalent bonds in the nitro group making it vulnerable to attack by hydrogen.
Step 3: formation of an amide
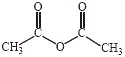
With the exception of tertiary amines, amines undergo reaction with anhydrides to produce amides.
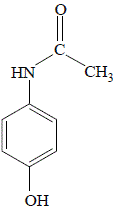
4-aminophenol, an amine, suspended in water at room temperature readily reacts with ethanoic anhydride (acetic anhydride) to produce a precipitate of the amide paracetamol (acetaminophen) as shown below:
| amine | acetic anhydride | amide |
 |
→
room temperature water |
 |
| 4-aminophenol |
|
paracetamol
(acetaminophen) |
Reactions of Paracetamol (acetaminophen)
Acid Hydrolysis
Hydrolysis (reaction with water) of amides in acidic solution produces an amine and a carboxylic acid.
Hydrolysis of paracetamol (acetaminophen) in acidic solution produces an amine (4-aminophenol) and a carboxylic acid (acetic acid)
| amide |
|
amine |
|
carboxylic acid |
 |
H2O/H2SO4
→
|
 |
+ |
|
paracetamol
(acetaminophen) |
|
4-aminophenol |
|
acetic acid
(ethanoic acid) |
1Paracetamol was marketed as Panadol by Sterling-Winthrop Co in 1953 in the UK.
2Paracetamol was marketed as Tylenol by McNeil Laboratories in 1955 in the USA.
3At 25oC pKa = 9.51
4This is only a general 'rule of thumb' but it is useful because it can be difficult to determine whether a carbon atom has 'gained' or 'lost' electrons.