Paper Chromatography Chemistry Tutorial
Key Concepts
- Paper chromatography is an analytical technique used to separate a mixture into its components.
- The stationary phase is usually the water present in the cellulose fibres of the paper1.
- The mobile phase refers to the solvent that moves up the paper by capillary action.
- Components in a mixture are separated based on their different abilities to bind or adsorb to the stationary phase, and on their different abilities to desorb or dissolve in the mobile solvent phase.
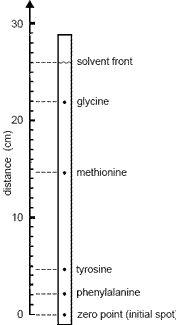
- The retardation factor, Rf, is a comparison of the distance travelled by a component to the distance travelled by the mobile solvent.
Rf = distance component travelled
distance solvent travelled - Rf values depend on temperature, quality of the paper used and the composition of the solvent used.
For these reasons, it is usual to run samples of known substances at the same time as you run the sample of the unknown mixture.