Classification of Organic Reactions Introductory Chemistry Tutorial
Key Concepts
- Organic compounds are, in general, compounds that contain carbon (C). (1)
- The reactions that organic compounds take part in can be classified based on what happens to the functional group.(2)
- The major types of reactions that organic compounds take part in are(3):
⚛ Substitution reactions: one atom substitutes for another
⚛ Addition reactions: a molecule adds across a double bond
⚛ Elimination reactions
(i) Dehydration reactions: elimination of water to produce a double bond
(ii) Condensation reactions: elimination of water when two molecules react
(iii) Dehydrohalogenation reactions: elimination of hydrogen halide from a molecule to produce a double bond
⚛ Oxidation reactions: addition of oxygen, or removal of hydrogen, using an oxidising agent
⚛ Reduction reactions: addition of hydrogen, or removal of oxygen, using H2 or a reducing agent
⚛ Polymerisation reactions:
(i) Addition polymerisation reactions: long chains formed when double bonds "open up"
(ii) Condensation polymerisation reactions: long chains formed when functional groups react
- It is important to be able recognise each type of reaction because it will help you understand how to approach the synthesis of an organic compound.
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Substitution Reactions
Alkanes do not react with acids, bases, oxidising agents nor reducing agents, but under the right conditions they will react with halogens in substitution reactions.
In a substitution reaction, one group on the molecule is replaced by another.
Imagine we have an organic compound containing a group labelled X :
which we react with the group labelled Y:
| |
|
|
H
| |
|
H
| |
|
| Y |
+ |
H− |
C |
− |
C |
−X |
| |
|
|
|
H |
|
|
H |
|
Y replaces X to make a new compound:
| |
|
|
|
H
| |
|
H
| |
|
|
|
|
H
| |
|
H
| |
|
|
| Y |
+ |
H− |
C |
− |
C |
−X |
→ |
X + |
H− |
C |
− |
C |
−Y |
| |
|
|
|
|
H |
|
|
H |
|
|
|
|
|
H |
|
|
H |
|
This is called a substitution reaction because the new group Y has been substituted for, or replaced, the original group X.
Consider the following chemical reaction:
This is a substitution reaction because a Br atom has replaced a H atom on the organic molecule.
Alkanes and haloalkanes (halogenoalkanes or alkyl halides) can undergo substitution reactions.
The table below summarises the subsitution reactions that you should become familiar with:
| Examples of Substitution Reactions |
|---|
| Description |
Example |
Reason for Classification as a Substitution Reaction |
|---|
| alkane → haloalkane |
H3C−CH3 + Br2 → H3C−CH2Br + HBr |
A Br atom has replaced an H atom on the organic molecule. |
| H3C−CH3 + Cl2 → H3C−CH2Cl + HCl |
A Cl atom has replaced an H atom on the organic molecule. |
| haloalkane → alkanol |
H3C−CH2Br + NaOH → H3C−CH2OH + NaBr |
An OH functional group has replaced the Br atom on the organic molecule. |
| haloalkane → ester |
H3C−CH2Br + CH3COO-Na+ → CH3−COO−CH2−CH3 + NaBr |
A CH3COO group has replaced the Br atom on the organic molecule. |
| haloalkane → alkanaminium halide |
H3C−CH2Br + NH3 → H3C−CH2NH3+ + Br- |
An NH3 functional group has replaced the Br atom on the organic molecule. |
| haloalkane → alkanenitrile |
H3C−CH2Br + KCN → H3C−CH2CN + KBr |
A CN functional group has replaced the Br atom on the organic molecule. |
Addition Reactions
In an addition reaction, a molecule "adds" across the double bond in the organic molecule.
Industrial ethanol (CH3CH2OH), for example, is produced from ethene (CH2=CH2), a product of the petroleum industry, in an addition reaction.
Imagine the following unsaturated organic molecule (unsaturated because it contains a double bond between 2 carbon atoms, C=C):
If we react this with a reagent X−Y so that we break the double bond (C=C) and make two new covalent bonds (C-X and C-Y) then this is an addition reaction as shown below:
Note that the parent hydrocarbon has been changed from an unsaturated alkene to a saturated alkane.
Consider the following chemical reaction of an unsaturated organic molecule with Br2 (represented below as Br−Br):
|
|
+ Br−Br → |
| |
Br
| |
|
Br
| |
|
| H− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
This is an addition reaction because Br2 (Br−Br) has "added" across the double bond (C=C).
The double bond (C=C) has become a single bond (C−C).
One of those carbon atoms has made a new covalent bond to one Br atom (C−Br), and the other carbon atom has made a new covalent bond to the other Br atom (C−Br).
The table below summarises the addition reactions that you should become familiar with:
| Examples of Addition Reactions |
|---|
| Description |
Example |
Reason for Classification as an Addition Reaction |
|---|
| alkene → dihaloalkane |
H2C=CH2 + Br2 → BrH2C−CH2Br |
Br2 has added across the double bond (C=C) |
| alkene → haloalkane |
H2C=CH2 + H−Br → H3C−CH2Br |
HBr has added across the double bond (C=C) |
| alkene → alkanol |
H2C=CH2 + H−OH → H3C−CH2OH |
Water, H2O, H−OH, has added across the double bond (C=C) |
Elimination Reactions
In an elimination reaction, a small molecule is eliminated, that is removed, from the reactant (or reactants) during the chemical reaction.
We will consider 3 different types of elimination reaction:
- Dehydration reactions: a saturated molecule reacts with a dehydrating agent to produce an unsaturated molecule and water.
- Condensation reactions: the functional group on one organic reactant reacts with the functional group on another organic molecule to produce a new compound and water.
- Dehydrohalogenation reactions: hydrogen halide is eliminated from a saturated haloalkane to produce one or more unsaturated organic products.
Dehydration Reactions
A dehydration reaction will convert a saturated organic molecule into an unsaturated molecule by eliminating, or removing, a water molecule.
Consider the reaction shown below:
This is a dehydration reaction, a type of elimination reaction, because a small molecule, water (H2O), has been eliminated from the saturated organic molecule (C−C) to produce an unsaturated molecule, a molecule that contains the double bond (C=C).
| Examples of Dehydration Reactions |
|---|
| Description |
Example |
Reason for Classification as an Elimination Reaction |
|---|
| Dehydration of Alkanols |
CH3−CH2OH → H2C=CH2 + H2O |
An H atom and the OH functional group have been eliminated from the molecule resulting in the formation of a double bond (C=C) |
Condensation Reactions
In a condensation reaction the functional group of one organic molecule reacts with the functional group of another organic molecule to produce a new organic molecule and water (H2O).
Condensation reactions are important in nature.
Disaccharides, such as sucrose (table sugar), are produced when monosaccharides react in condensation reactions.
Condensation reactions can also be used to produce esters, one of the groups of compounds that makes up the odours and flavours of food.
Consider the reaction below in which 2 molecules with different functional groups react :
This is a condensation reaction, a type of elimination reaction, because the OH functional group of one molecule has reacted with the COOH functional group of a different molecule to produce a new organic compound and a molecule of water (H2O).
The table below summarises some of the condensation reactions you should become familiar with.
| Examples of Condensation Reactions |
|---|
| Description |
Example |
Reason for Classification as an Elimination Reaction |
|---|
| alkanoic acid + alkanol |
CH3−COOH + HOCH2−CH3 → CH3−COOCH2−CH3 + H2O |
An H atom from one molecule's OH functional group and an OH from the other molecule's COOH functional group have been eliminated when the two molecules are joined together and a molecule of water is produced. |
| amino acids → dipeptides |
H2N-R-COOH + H2N-R'-COOH → H2N-R-CO-NH-R'-COOH + H2O |
An H atom from one molecule's NH2 functional group and an OH from the other molecule's COOH functional group have been eliminated when the two molecules are joined together and a molecule of water is produced. |
| monosachharides → disaccharides |
|
An H atom from one molecule's OH functional group and an OH from the other molecule's OH functional group have been eliminated when the two molecules are joined together and a molecule of water is produced. |
Dehydrohalogenation Reactions
In a dehydrohalogenation reaction a hydrogen halide (HX) is eliminated from a saturated haloalkane to produce new unsaturated organic products.
For example, hydrogen chloride can be eliminated from 2-chloropropane to produce prop-1-ene:
| |
H
| |
|
Cl
| |
|
H
| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
→ |
| H |
|
|
|
|
H |
|
| |
\ |
|
|
|
| |
|
| |
C |
= |
C |
− |
C |
−H |
| |
/ |
|
| |
|
| |
|
| H |
|
|
H |
|
H |
|
|
+ HCl |
| Examples of Dehydrohalogenation Reactions |
|---|
| Description |
Example |
Reason for Classification as an Elimination Reaction |
|---|
| haloalkane → alkene |
CH3−CHCl−CH3 → CH2=CH-CH3 + HCl |
An H atom from a methyl group (CH3) and the Cl functional group have been removed, eliminated, from the molecule as a molecule of hydrogen chloride, HCl. |
Oxidation Reactions
Oxidation reactions are the main reason that food goes "off", wine turns to vinegar and butter becomes rancid.
A general "rule of thumb" for organic reactions is that an oxidation reaction has occurred if:(4)
- oxygen atoms have been added to the organic reactant to form the product
or
- hydrogen atoms have been removed from the organic reactant to form the product
Typically an inorganic oxidising agent, such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7), is used to oxidise an organic molecule.
The presence of an oxidising agent may be indicated by the use of an O surrounded by square brackets, [O], above the arrow showing the direction of the reaction (→).
Consider the following chemical reaction:
| |
H
| |
|
H
| |
|
O
|| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
|
|
[O]
→ |
| |
H
| |
|
H
| |
|
O
|| |
| H− |
C |
− |
C |
− |
C |
−OH |
| |
|
H |
|
|
H |
|
|
|
|
This is an example of an oxidation reaction because an oxidising agent, [O], has caused an increase in the number of O atoms in the organic product compared to the organic reactant molecule.
There is 1 O atom in the reactant molecule but 2 O atoms in the product molecule.
Consider the following chemical reaction:
| |
H
| |
|
OH
| |
H
| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
H |
|
|
H |
|
|
[O]
→ |
| |
H
| |
|
O
|| |
H
| |
|
| H− |
C |
− |
C |
− |
C |
−H |
| |
|
H |
|
|
|
|
H |
|
|
This reaction is an oxidation reaction because an oxidising agent, [O], has caused the removal of 2 H atoms from the reactant molecule in order to produce the product molecule.
There are 8 H atoms in the reactant molecule but only 6 H atoms in the product molecule.
The table below summarises some of the oxidation reactions you should become familiar with.
| Examples of Oxidation Reactions |
|---|
| Description |
Example |
Reason for Classification as an Oxidation Reaction |
|---|
| alkene → alkanediols |
H2C=CH2 → CH2OH−CH2OH |
2 O atoms have been added to the reactant molecule to form the product. |
| 1° alkanol → alkanal |
H3C−CH2OH → H3C−CHO |
2 H atoms have been lost from the reactant molecule to form the product molecule. |
| alkanal → alkanoic acid |
H3C−CHO → H3C−COOH |
An O atom has been added to the reactant molecule to form the product molecule. |
| 2° alkanol → alkanone |
H3C−CHOH−CH3 → H3C−CO−CH3 |
2 H atoms have been lost from the reactant molecule to form the product molecule. |
Reduction Reactions
A general "rule of thumb" for organic reactions is that a reduction reaction has occurred if:(5)
- hydrogen atoms have been added to the organic reactant to form the product
or
- oxygen atoms have been removed from the organic reactant to form the product
Typically, hydrogen gas (H2) or an inorganic reducing agent is used to reduce an organic molecule.
We can use an H surrounded by square brackets, [H], above the arrow indicating the direction of the reaction (→) to represent the use of a reducing agent.
When hydrogen gas is used (with a catalyst) the reaction is usually referred to as a hydrogenation reaction.
In the laboratory, reduction reactions are useful when you want to synthesise an amine.
Consider the following reaction:
This is a reduction reaction because a reducing agent has caused the addition of 2 H atoms to the reactant molecule.
The reactant molecule has 4 H atoms, the product molecule has 6 H atoms.
Consider the chemical reaction below:
This is a reduction reaction because an O has been removed and 2 H atoms have been added to the reactant molecule to form the product.
The reactant molecule has 2 O atoms and 4 H atoms, the product molecule has 1 O atom and 6 H atoms.
The table below summarises some of the reduction reactions you should become familiar with.
| Examples of Reduction Reactions |
|---|
| Description |
Example |
Reason for Classification as a Reduction Reaction |
|---|
| alkene → alkane |
H2C=CH2 → H3C−CH3 |
2 H atoms have been added to the reactant molecule to form the product molecule. |
| alkanal → alkan-1-ol |
H3C−CHO → H3C−CH2OH |
2 H atoms have been added to the reactant molecule to form the product molecule. |
| alkan-n-one → alkan-n-ol |
H3C−CO−CH3 → H3C−CHOH−CH3 |
2 H atoms have been added to the reactant molecule to form the product molecule. |
| alkanenitrile → alkanamine |
H3C−C≡N → H3C−CH2−NH2 |
4 H atoms have been added to the reactant molecule to form the product molecule. |
Polymerisation Reactions
Polymerisation reactions are important because the products produced (polymers) are used to make synthetic fibres like nylon and polyester, and plastics like polyethylene (polyethene) and polytetrafluorethene (Teflon™).
In a polymerisation reaction, molecules with a small number of carbon atoms (monomers) react to produce long chains of carbon atoms (polymers).
There are 2 types of polymersation reactios:
- Addition polymerisation reactions
- Condensation polymerisation reactions
Addition Polymerisation Reactions
Addition polymerisation is an important industrial process because it is used to produce the polymers that are present in "plastics".
These polymers include polyethene, polyvinyl chloride (PVC), polypropylene, polystyrene, polytetrafluoroethene (Teflon), polyacrylonitrile and polyvinylacetate.
In an addition polymerisation reaction, the reactant molecule (monomer) is unsaturated (contains a double bond, C=C).
This double bond "opens up" so that this molecule can covalently bond to another molecule, which can itself bond to another molecule, and so on.
In this fashion long chains of covalently bonded carbon atoms can be produced.
This long chain of carbon atoms is called a polymer.
Consider the following polymerisation reaction involving H2C=CH2 monomers.
--- indicates that the chain keeps going on in the same fashion.
|
|
→ |
| |
H
| |
|
H
| |
|
H
| |
|
H
| |
|
| --- |
C |
− |
C |
− |
C |
− |
C |
--- |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
This is a polymerisation because small molecules have joined together to produce extremely long chains of carbon atoms.
This is an addition polymerisation because the double bond in the reactant molecule, C=C, has "opened up" allowing other small molecules to "add on" to the growing chain.
The table below summarises some addition polymerisation reactions you should become familiar with.
| Examples of Addition Polymerisation Reactions |
|---|
| Description |
Example |
Reason for Classification as an Addition Polymerisation Reaction |
|---|
| alkene → polyalkene |
nCH2=CH2 → -[CH2−CH2]n- |
C=C double bond in each CH2=CH2 molecule has opened up to allow them to join together to form a long chain. |
| haloalkene → polyhaloalkene |
nCH2=CHCl → -[CH2−CHCl]n- |
C=C double bond in each CH2=CHCl molecule has opened up to allow them to join together to form a long chain. |
| tetrahaloalkene → polytetrahaloalkene |
nCF2=CF2 → -[CF2−CF2]n- |
C=C double bond in each CF2=CF2 molecule has opened up to allow them to join together to form a long chain. |
Condensation Polymerisation Reactions
Condensation polymerisation reactions are important in nature.
Proteins are polymers formed when amino acids monomers react in condensation polymerisation reactions.
Polysaccharides such as starch and cellulose are produced when monosaccharide monomers react in condensation polymerisation reactions.
In a condensation polymerisation reaction, each small monomer molecule has 2 functional groups, lets call them X and Y:
Let's imagine that each X functional group can react with a Y functional group so that a molecule XY is eliminated, and the two organic molecules join together:
|
|
+ |
|
→ |
| |
H
| |
|
H
| |
|
H
| |
|
H
| |
|
| X− |
C |
− |
C |
− |
C |
− |
C |
−Y |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
|
+ |
X−Y |
But the X functional group on this new chain could react with the Y functional group of a monomer or of another new chain.
And the Y functional group on this chain could react with the X functional group of a monomer or of another chain.
So it is easy to see how very longs of polymer could be produced.
If the small molecule being eliminated is a water molecule, that is, if X−Y is H2O, then this would be referred to as a condensation polymerisation reaction.
| Examples of Condensation Polymerisation Reactions |
|---|
| Description |
Example |
Reason for Classification as a Condensation Polymerisation Reaction |
|---|
| dicarboxylic acid + diol → polyester |
HOOC-R-COOH + CH2OH-R'-CH2OH → [-O-CO-R-CO-O-CH2-R'-CH2O]n + (n-1)H2O |
COOH functional group of one molecule reacts with OH functional group of another molecule to produce a new product and a molecule of water. The new organic molecule can then react with either of the functional groups of another monomer, or of a new chain, so that the chain continues to grow (polymerise). |
| dicarboxylic acid + diamine → polyamide |
HOOC-R-COOH + NH2-R'-NH2 → [CO-R-CO-NH-R'-NH]n + (n-1)H2O |
The COOH functional group of one molecule reacts with the NH2 functional group of another molecule to produce a new organic product and a molecule of water. The functional groups of this new product can react with the functional groups of either monomer, or a new product molecule, to produce longer and longer chains of carbon atoms. |
| monosachharides → polysaccharides |
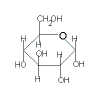 |
→ |
 |
+ xH2O |
|
An H atom from one molecule's OH functional group and an OH from the other molecule's OH functional group have been eliminated when the two molecules are joined together and a molecule of water is produced. |
| amino acids → proteins |
H2N-R-COOH + H2N-R'-COOH → [HN-R-CO-NH-R'-CO]n + xH2O |
An H atom from one molecule's NH2 functional group and an OH from the other molecule's COOH functional group have been eliminated when the two molecules are joined together and a molecule of water is produced. Further reactions between these 2 functional groups on different molecules produce longer and longer chains. |
(1) This is a general definition of organic compounds. There are many compounds that contain carbon that are generally classified as inorganic rather than organic, for example, carbon dioxide (CO2) and carbon monoxide (CO), as well the carbonate ion and carbonate compounds (CO32-), even hydrogen cyanide (HCN) and cyanide salts (CN-).
(2) These reactions can be further sub-classified on the basis of the nature of the attacking agent, that is whether it is electrophilic or nucleophilic, but since this is not generally covered in high school chemistry in Australia we will ignore it.
(3) Another type of organic reaction is the rearrangement, but this is rarely considered in High School so we will ignore it.
(4) A better description of an oxidation reaction is that the use of an oxidising agent causes an increase in carbon electrovalence in the organic molecule.
(5) A better description of a reduction reaction is that the use of either a reducing agent or catalytic hydrogenation causes a decrease in carbon electrovalence in the organic molecule.




