Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Omega Classification of Fatty Acids
Fatty acids are long chain carboxylic acids.
Fatty acids contain the COOH (carboxyl) functional group at the end of a long hydrocarbon chain.
Fatty acids can be classified as:
- saturated (only single bonds between carbon atoms)
or,
- unsaturated (containing one or more double bonds between carbon atoms).
Some examples of saturated and unsaturated fatty acids are give below:
| |
Some Fatty Acids |
|---|
| |
Trivial
Name |
Molecular
Formula |
Semi-Structural Formula
(condensed structural formula) |
|---|
Saturated
fatty acids |
lauric acid |
C11H23COOH |
CH3(CH2)10COOH |
|---|
| myristic acid |
C13H27COOH |
CH3(CH2)12COOH |
| palmitic acid |
C15H31COOH |
CH3(CH2)14COOH |
| stearic acid |
C17H35COOH |
CH3(CH2)16COOH |
| arachidic acid |
C19H39COOH |
CH3(CH2)18COOH |
Unsaturated
fatty acids |
palmitoleic acid |
C15H29COOH |
CH3(CH2)4CH2CH=CHCH2(CH2)5CH2COOH |
|---|
| oleic acid |
C17H33COOH |
CH3(CH2)7CH=CH(CH2)7COOH |
| linoleic acid |
C17H31COOH |
CH3(CH2)4(CH=CHCH2)2(CH2)6COOH |
| linolenic acid |
C17H29COOH |
CH3CH2(CH=CHCH2)3(CH2)6COOH |
| arachidonic acid |
C19H31COOH |
CH3(CH2)4(CH=CHCH2)3CH=CH(CH2)3COOH |
Using the IUPAC recommendations for naming carboxylic acids, Chemists number the carbon chain of a fatty acid starting from the carbon of carboxyl, COOH, functional group as 1.
The structural formula for palmitoleic acid is shown below with the carbons numbered from 1 to 16 according to IUPAC recommendations:
| |
CH3 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
IUPAC
(chemistry) |
16 |
|
15 |
|
14 |
|
13 |
|
12 |
|
11 |
|
10 |
|
9 |
|
8 |
|
7 |
|
6 |
|
5 |
|
4 |
|
3 |
|
2 |
|
1 |
|
and we note that the double bond occurs between carbons 9 and 10 in the hydrocarbon chain.
Chemists classify palmitoleic acid as:
- a carboxylic acid (it has the COOH functional group)
- unsaturated (it has a C=C)
- monounsaturated (it has only ONE C=C)
- a fatty acid (long chain carboxylic acid)
There is another way we could classify fatty acids which is based on the location of the double bond.
Biologists, especially biochemists, commonly classify fatty acids this way.
To do this they label the carbon adjacent to the carboxyl group the alpha carbon, α carbon, (alpha being the first letter of the Greek alphabet), and the last carbon atom in the hydrocarbon chain as the omega carbon atom, ω carbon (omega being the last letter of the Greek alphabet) as shown on the structural formula of palmitoleic acid below:
| |
CH3 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
Biological
classification |
ω |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α |
|
|
|
Then they count back from the omega carbon atom (omega−1, or, ω−1) towards the alpha carbon atom until they hit upon the first double-bonded carbon atom as shown below:
| |
CH3 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
Biological
classification |
ω−1 |
|
ω−2 |
|
ω−3 |
|
ω−4 |
|
ω−5 |
|
ω−6 |
|
ω−7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α |
|
|
|
Palmitoleic acid, shown above, would then be classified as an omega−7 (ω−7) fatty acid.
The double bond occurs between the 7th and 8th carbon atoms as counted from the end of the hydrocarbon chain.
Consider oleic acid, another monounsaturated fatty acid:
| |
CH3 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
| |
ω−1 |
|
ω−2 |
|
ω−3 |
|
ω−4 |
|
ω−5 |
|
ω−6 |
|
ω−7 |
|
ω−8 |
|
ω−9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α |
|
|
|
Oleic acid would be classified as an omega−9 (ω−9) fatty acid.
Consider linoleic acid, a polyunsaturated fatty acid (it has two lots of C=C):
| |
CH3 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
| |
ω−1 |
|
ω−2 |
|
ω−3 |
|
ω−4 |
|
ω−5 |
|
ω−6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α |
|
|
|
Linoleic acid would be classified as an omega−6 (ω−6) fatty acid.
Consider linolenic acid, another polyunsaturated fatty acid (it has 3 lots of C=C):
| |
CH3 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
| |
ω−1 |
|
ω−2 |
|
ω−3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α |
|
|
|
Linolenic acid is an omega−3 (ω−3) fatty acid.
Consider arachidonic acid, another polyunsaturated fatty acid (it has 4 lots of C=C):
| |
CH3 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH |
= |
CH |
- |
CH2 |
- |
CH2 |
- |
CH2 |
- |
COOH |
|
| |
ω−1 |
|
ω−2 |
|
ω−3 |
|
ω−4 |
|
ω−5 |
|
ω−6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
α |
|
|
|
Arachidonic acid is an omega−6 (ω−6) fatty acid.
This omega classification can only be given to unsaturated fatty acids, that is, the fatty acid MUST contain one or more double bonds between carbon atoms.
The omega classification does not tell us how many double bonds there are, nor does it tell us how long the chain is, it only tells us the location of the first double bond as counted from the end of the hydrocarbon chain.
Omega−3 Fatty Acids and Omega−6 Fatty Acids
Omega−3 fatty acids and omega−6 fatty acids are different classes of fatty acids.
The human body lacks the ability to introduce a double bond between carbons 9 and 10 and beyond, as counted from the carboxyl functional group (IUPAC nomenclature for chemistry), so fatty acids with double bonds beyond the ninth carbon atom are essential fatty acids because we must eat foods containing them.
Two essential fatty acids are linoleic acid and alpha-linolenic acid (α-linolenic acid).
α-linolenic acid, C18H30O2, is an example of an omega−3 fatty acid (ω−3 fatty acid).
According to IUPAC nomenclature rules, the carbon of the carboxyl group is given the number 1, the carbon atom next to that is 2, and so on and so forth until the final carbon atom is numbered 18.
Numbering according to IUPAC rules is shown as blue numbers on the skeletal structural formula shown below:
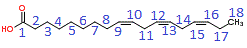
The carbon chain is 18 carbon atoms long, which, according to IUPAC nomenclature makes the parent carbon chain "octadeca".
The location of each of the three (tri) double bonds (en) are on carbons 9, 12 and 15, that is 9,12,15-trien
The functional group is COOH, or a carboxyl group, and since there are no other functional groups present this is named as an alkenoic acid, with the oic acid suffix
The completed IUPAC name(2) is octadeca-9,12,15-trienoic acid or 9,12,15-octadecatrienoic acid
α-linolenic acid is an omega-3 fatty acid because, when counted from the last carbon atom in the chain (omega carbon atom, 1), the first C=C encounted occurs at the third carbon atom along from the omega carbon atom:

Linoleic acid, C18H32O2, on the other hand, is an example of omega-6 fatty acid (ω-6 fatty acid).
According to IUPAC nomenclature rules, the carbon of the carboxyl group is given the number 1, the carbon atom next to that is 2, and so on and so forth until the final carbon atom is numbered 18.
Numbering according to IUPAC rules is shown as blue numbers on the skeletal structural formula shown below:
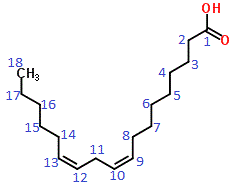
The carbon chain is 18 carbon atoms long, which, according to IUPAC nomenclature makes the parent carbon chain "octadeca".
The location of each of the two (di) double bonds (en) are on carbons 9 and 12, that is 9,12-dien
The functional group is COOH, or a carboxyl group, and since there are no other functional groups present this is named as an alkenoic acid, with the oic acid suffix
The completed IUPAC name(3) is octadeca-9,12-dienoic acid or 9,12-octadecadienoic acid
Linoleic acid is an example of an omega-6 fatty acid because, when counted from the omega carbon atom (last carbon atom of the chain), the first C=C occurs at the sixth carbon atom in the chain as shown below:
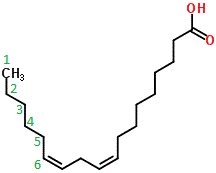
Note that the double bonds in the carbon skeleton of omega−3 and omega−6 fatty acids introduces kinks in the 3-dimensional arrangement.
These kinks prevent the fatty acid molecules from packing together closely, reducing the strength of the intermolecular forces acting between them, thereby reducing their melting points (as compared to a saturated fatty acid with the same hydrocarbon chain length).
For this reason, we find that omega−3 and omega−6 fatty acids are liquids (oils) rather than solids (fats) at room temperature and pressure.
These omega−3 and omega−6 fatty acids are found in such foods as:
- fish oil
- vegetable oils such as olive oil and canola oil
- seeds such as chia, pumpkin and sunflower seeds
Footnotes:
(1) A better definition might be that a fatty acid is the carboxylic acid product produced from the hydrolysis of a triglyceride.
In general, this product is a carboxylic acid with a long, unbranched carbon chain.
Hence, a fatty acid can be defined as a long chain carboxylic acid.
The definitions above work well for chemistry students, but work less well for biology students who will have to deal with the following terms:
(a) short chain fatty acids, SCFA, (literally short chain long chain fatty acids?)
(b) medium chain fatty acids, MCFA, (literally medium chain long chain fatty acids?)
(c) long chain fatty acids, LCFA, (literally long chain long chain fatty acids?)
(2) It might also be noted that each of the double bonds has a cis geometry, or Z configuration in absolute geometry, so a better IUPAC name is (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid
(3) It might also be noted that each of the double bonds has a cis geometry, or Z configuration in absolute geometry, so a better IUPAC name is (9Z,12Z)-octadeca-9,12-dienoic acid


