Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Theory Behind the Experimental Determination of the Empirical Formula of Magnesium Oxide
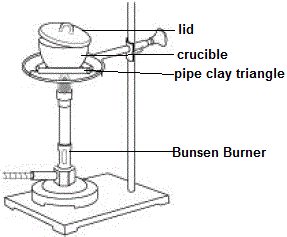
Magnesium metal reacts with oxygen from the atmosphere in a combustion reaction to produce grey-white solid magnesium oxide.
magnesium + oxygen gas → magnesium oxide
Since the product, magnesium oxide, contains only magnesium "atoms" and oxygen "atoms"(1), we could write the formula MgxOy in which:
x represents the number of Mg "atoms"
y represents the number of O "atoms"
We cannot see the atoms of each element in the compound because they are much too small, so we can't count them just be looking at 1 "molecule" of magnesium oxide. We need a different way to determine the values of x and y.
There is a relationship between the mass of an element and the number of "atoms" of that element:
6.02 × 1023 atoms of an element has a mass equal to its atomic weight expressed in grams
The atomic weight of each element is listed in the Periodic Table of the Elements:
atomic weight of magnesium (Mg) = 24.31
atomic weight of oxygen (O) = 16.00
Therefore, if we know the mass of an element we can calculate how many atoms of that element are present:
mass of 6.02 × 1023 atoms of an element = element's atomic weight expressed in grams (molar mass)
mass of 1 atom of an element in grams = molar mass ÷ 6.02 × 1023
| mass of z atoms of an element in grams |
= |
z × molar mass
6.02 × 1023 |
| mass of z atoms of an element in grams × 6.02 × 1023 |
= |
z × molar mass |
mass of z atoms of an element in grams × 6.02 × 1023
molar mass |
= |
z |
So, if we know the mass of magnesium and the mass of oxygen making up our sample of magnesium oxide, MgxOy, product, then:
the number of magnesium atoms in a given mass of magnesium atoms can be calculated:
| x |
= number of magnesium atoms |
= |
mass of the magnesium in grams × 6.02 × 1023
24.31 |
and, the number of oxygen atoms in a given mass of oxygen atoms can be calculated:
| y |
= number of atoms of oxygen |
= |
mass of the oxygen atoms in grams × 6.02 × 1023
16.00 |
In this experiment we will determine the empirical formula for magnesium oxide, that is, we will determine the lowest whole number ratio of x to y:
| x |
: |
y |
| number of magnesium atoms |
: |
number of oxygen atoms |
mass of the magnesium atoms in grams × 6.02 × 1023
24.31 |
: |
mass of the oxygen atoms in grams × 6.02 × 1023
16.00 |
mass of the magnesium atoms in grams × 6.02 × 1023
24.31 × 6.02 × 1023 |
: |
mass of the oxygen atoms in grams × 6.02 × 1023
16.00 × 6.02 × 1023 |
mass of the magnesium atoms in grams
24.31 |
: |
mass of the oxygen atoms in grams
16.00 |
Note that the quantity equal to an element's mass divided by its molar mass is measured in units of moles:
| x |
: |
y |
mass of the magnesium atoms in grams
24.31 |
: |
mass of the oxygen atoms in grams
16.00 |
| moles of the magnesium atoms |
: |
moles of the oxygen atoms |
So, we only need to measure the mass of magnesium and the mass of oxygen present in the magnesium oxide sample in order to determine the ratio of moles of magnesium to moles of oxygen, from which we can determine the empirical formula of the magnesium oxide.
The Law of Mass Conservation tells us that during a chemical reaction mass can neither be created nor destroyed, so the total mass of the system before the chemical reaction must be equal to the total mass of the system after completion of the chemical reaction:
| magnesium metal |
+ |
oxygen gas |
→ |
magnesium oxide solid |
| mass of all reactants |
= |
mass product |
Magnesium metal and magnesium oxide are both solids at room temperature and pressure, so we can easily weigh these in order to determine their mass.
We can not easily weigh the amount of oxygen gas used to combust the magnesium, but we don't have to because we can use the Law of Mass Conservation to calculate how much oxygen is present in the magnesium oxide we produce:
mass oxygen in compound = (mass magnesium oxide) - (mass magnesium used)
The mass of magnesium used and the mass of oxygen atoms we calculate can then be used to determine the ratio of magnesium atoms to oxygen atoms in the compound using the relationship we derived above, that is:
| x |
: |
y |
| number of magnesium atoms |
: |
number of oxygen atoms |
mass of the magnesium atoms in grams
24.31 |
: |
mass of the oxygen atoms in grams
16.00 |
However, the value of x and the value of y will probably be fractions (or decimals) rather than whole numbers:
for example x : y is calculated to be 0.072 : 0.069
In order to force this into a ratio of whole numbers we will divide both x and y by the lowest number (0.069 in this example):
| |
x |
: |
y |
|---|
| calculated values |
0.072 |
: |
0.069 |
|---|
| divide both by lowest number |
0.072 ÷ 0.069 |
: |
0.069 ÷ 0.069 |
|---|
| gives a new ratio |
1.04 |
: |
1.00 |
|---|
| 1.04 ≈ 1 so |
1 |
: |
1 |
|---|
We then write the empirical formula for the magnesium oxide (MgxOy) by replacing the x and y with the values we have calculated, for example:
| x |
1 |
1 |
2 |
2 |
3 |
|---|
| y |
1 |
2 |
1 |
3 |
2 |
|---|
empirical formula
(MgxOy) |
MgO |
MgO2 |
Mg2O |
Mg2O3 |
Mg3O2 |
|---|
Note that if either x=1 or y=1 then the subscript 1 is not written in the formula, that is, Mg1O2 is actually written as MgO2, and, Mg2O1 would only ever be written as Mg2O.