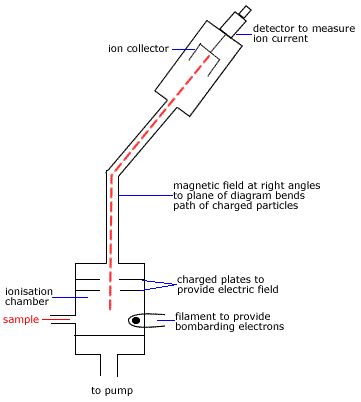
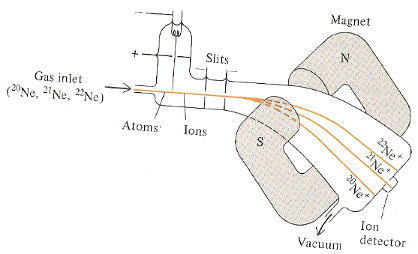
Example: Using a Mass Spectrum to Determine an Approximate Relative Atomic Mass of an Element
The mass spectrum could be presented in two ways, either giving the actual signal intensity for each isotope, or, giving you the relative peak heights or percentage abundances.
| Signal Intensity Mass Spectrum of Neon | % Abundance Mass Spectrum of Neon |
 |
|
Relative atomic mass (r.a.m.) = [(%abundance isotope a × mass isotope a) + (%abundance isotope b × mass isotope b) +(%abundance isotope c × mass isotope c)] ÷ 100
Mass number (A) can be used to approximate the mass of an isotope.
(see Relative Atomic Mass tutorial for details)
For the isotopes of neon:
the mass of 20Ne ≈ A(neon-20) ≈ 20
the mass of 21Ne ≈ A(neon-21) ≈ 21
the mass of 22Ne ≈ A(neon-22) ≈ 22
So the calculation for relative atomic mass (or atomic weight), now looks like this:
Relative atomic mass (Neon) = [(%abundance neon-20 × A(neon-20)) + (%abundance neon-21 × A(neon-21)) +(%abundance neon-22 × A(neon-22))] ÷ 100
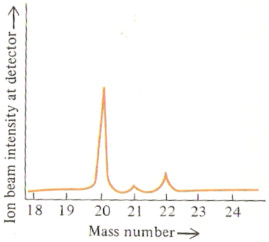
The % abundance of each isotope can be read directly off the second mass spectrum given above.
% abundance 20Ne = 90.92 %
% abundance 21Ne = 0.26 %
% abundance 22Ne = 8.82 %
Relative atomic mass (r.a.m.), or atomic weight, can then be calculated:
r.a.m. (Ne) = [(90.92 × 20) + (0.26 × 21) + (8.82 × 22)] ÷ 100
= [1818.4 + 5.46 + 194.04] ÷ 100
= 20.18
Example: Using a Mass Spectrum to Determine the Relative Atomic Mass of an Element
If the data you are provided with give you the actual mass2 of each isotope (isotopic mass) as well as (or instead of) the mass number of each isotope, then you should use the actual isotopic masses in your calculation of relative atomic mass (r.a.m.).
| Signal Intensity Mass Spectrum of Neon | Tabulated Data |
 |
| Mass Number | Isotopic Mass | % Abundance |
|---|
| 20 | 19.992 | 90.92 |
| 21 | 20.994 | 0.26 |
| 22 | 21.991 | 8.82 |
|
Relative atomic mass (r.a.m.) = [(%abundance neon-20 × mass neon-20) + (%abundance neon-21 × mass neon-21) +(%abundance neon-22 × mass neon-22)] ÷ 100
r.a.m. (Ne) = [(90.92 × 19.992) + (0.26 × 20.994) + (8.82 × 21.991)] ÷ 100
= [1817.6 + 5.4584 + 193.96] ÷ 100
= 20.17
1. Mass spectroscopy can also be used to determine the structure of molecules (Mass spectroscopy for structural determination)
2. The mass of an isotope is not a whole number because the measured mass of a proton and of a neutron and of an electron is not a whole number. (Find out more at Mass Defect)