Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Hydrolysis of Disaccharides
Sucrose, table sugar, is an example of a disaccharide.
It is produced by the condensation reaction between the monosaccharides glucose and fructose as shown below:
| glucose |
+ |
fructose |
→ |
sucrose |
+ water |
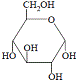 |
+ |
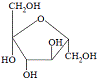 |
→ |
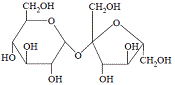 |
+ H2O(l) |
Notice the -C-O-C- (glycosidic link or ether bond) between the glucose unit and the fructose unit in a molecule of sucrose.
The reverse reaction is the hydrolysis of sucrose to produce glucose and fructose:
| sucrose |
H2O
→ |
glucose |
+ |
fructose |
 |
H2O
→ |
 |
+ |
 |
Notice that a water molecule has effectively been added across the glycosidic link resulting in the formation of two monosaccharides; glucose and fructose.
In the laboratory we would use heat and acidic conditions to hydrolyse sucrose, but in your body you use an enzyme, sucrase, in an enzyme catalysed hydrolysis reaction.
Lactose, "milk sugar", is also a disaccharide that can undergo hydrolysis:
| lactose |
H2O
→ |
glucose |
+ |
galactose |
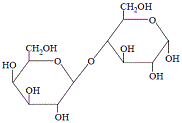 |
H2O
→ |
 |
+ |
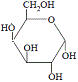 |
Notice that a water molecule has effectively been added across the glycosidic link resulting in the formation of two monosaccharides; glucose and galactose.
In the laboratory we would use heat and acidic conditions to hydrolyse lactose, but in your body you use an enzyme, lactase, in an enzyme catalysed hydrolysis reaction.
For every 1 molecule of disaccharide that undergoes hydrolysis, 2 molecules of monosachharide are produced.
| 1 disaccharide molecule |
H2O
→ |
2 monosaccharide molecules |
Hydrolysis of Polysaccharides
Amylose is a component of starch.
It is a polymer made up of many glucose units, that is, it is a polysaccharide.
The skeletal structure below shows 3 glucose units, but the square brackets with the subscript n means that there are n repeating units in the chain:
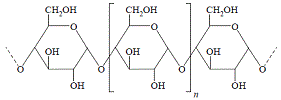
Notice the -C-O-C- (glycosidic links or ether bonds) between each glucose unit in the structure of amylose.
If we hydrolyse amylose so that we add water across all these glycosidic links (ether bonds) we end up with n+2 glucose molecules:
| amylose |
H2O
→ |
(n+2)glucose |
 |
H2O
→ |
(n+2) |
Note that if the hydrolysis of amylose does not go to completion you will have a mixture containing molecules of various chain lengths.
Maltose, for example, is a disaccharide made up of 2 units of glucose, and is the major disaccharide product of the digestion of starch in humans.
The disaccharide maltose can be broken down into glucose in a hydrolysis reaction catalysed by the enzyme maltase in your body.
In the laboratory we would hydrolyse amylose using heat and acidic conditions, but in your body you use an enzyme, amylase found in saliva, in an enzyme catalysed hydrolysis reaction.
Digestion of Carbohydrates in the Human Body
You eat carbohydrates such as sugars and starches to give you energy.
The digestion of carbohydrates by enzyme catalysed hydrolysis begins in your mouth and continues in your stomach and small intestine.
The final product of the digestion of carbohydrates are monosaccharides such as glucose and fructose.
Glucose is used by your cells during respiration to produce energy.
Any excess glucose that is not immediately required for cell respiration will be stored.
Some of the excess will be stored as fat in adipose tissue.
Some will undergo condensation polymerisation to produce glycogen which is stored in the liver.
When required, this glycogen undergoes enzyme catalysed hydrolysis to produce the glucose required for cell respiration.






