Latent Heat or Latent Enthalpy Chemistry Tutorial
Key Concepts
- Latent heat is also known as latent enthalpy.1
- Latent Heat (latent enthalpy) is the "hidden" heat when a substance absorbs or releases heat without producing a change in the temperature of the substance. 2
- While a substance changes state, its temperature does not change, even though heat energy is being absorbed, or released, by the substance.
This heat energy that is absorbed or released is known as the latent heat, or latent enthalpy, because it is "hidden", that is, not causing a change in temperature which we could measure.
- Latent Heat of Fusion (latent enthalpy of fusion) is the heat absorbed per mole when a substance changes state from solid to liquid at constant temperature (melting point).
- Latent Heat of Vaporization or vaporisation (latent enthalpy of vaporisation) is the heat absorbed per mole when a substance changes state from liquid to gas at constant temperature (boiling point).
- Latent Heat of Sublimation (latent enthalpy of sublimation) is the heat absorbed per mole when a substance changes state from solid to gas, without going through the liquid phase, at constant temperature.
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Heating Curves and Latent Heat
Have you ever noticed that, if you wake up in the morning while it is still dark and there is dew on the ground, when the sun begins to rise the air temperature seems to go down before it goes up?
It isn't your imagination playing tricks on you, it really does get colder before it gets warmer!
So where does all the heat energy go at sunrise?
You can try this experiment at home or at school.
All you need is a thermometer, some ice (solid water, H2O(s)), and a small beaker (or a small glass if you are at home).
- Suspend the thermometer in the beaker so that its bulb or reservoir at the bottom is a couple of millimeters from the base of the beaker, and gently clamp the thermometer in position.
- Add enough ice to cover the bulb or reservoir of the thermometer.
- Record the temperature and your observations every few minutes.
The results of your experiment might look like those in the table below:
Time
(minutes) |
Temperature
(°C) |
Observations |
|---|
| 0 |
0 |
Lots of ice, no liquid |
| 5 |
0 |
A little less ice, a little liquid |
| 10 |
0 |
Even less ice, more liquid |
| 15 |
0 |
Little ice left, lots of liquid |
| 20 |
5 |
No ice, all liquid water |
| 25 |
12 |
No ice, all liquid water |
The ice is melting so we know that heat energy is being absorbed by the solid ice to turn it into liquid water, but, the temperature remains constant while the ice melts.
Energy is being absorbed from the surroundings in order to melt the ice, BUT, this energy is "hidden" because it is not making itself observable as an increase in temperature.
We refer to this "hidden heat energy" as "latent heat" (or latent enthalpy).
Therefore we could refer to the latent heat used to melt the ice as its "latent heat of melting".
Chemists use the term "fusion" for the process of heating a solid, so the "hidden" heat to melt a solid is called the Latent Heat of Fusion (or latent enthalpy of fusion).
Once there is no ice left, the temperature of liquid water begins to increase, that is, the energy being absorbed by the liquid water is observable as an increase in temperature.
If you were to continue the experiment by heating the liquid water using a bunsen burner or heating mantle, you would find the temperature of the water will continue to increase until it starts boiling.
While there is liquid water to boil, the temperature of the water will remain constant.
If you were to plot the results of the experiment on a graph, you would produce a graph like the one shown below for a pure substance being heated at a uniform rate:
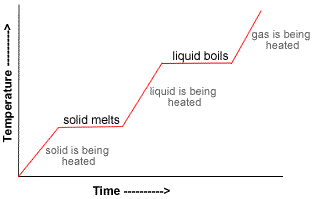
- Initially, the temperature of the solid rises uniformly until further heating does not increase the temperature.
At this constant temperature, the solid is melting.
This constant temperature is known as the melting point.
- At the melting point heat is absorbed and used to melt the solid without any temperature change (latent heat of fusion), all the energy is going into weakening the intermolecular forces between the particles in the solid.
- When all the solid has melted to a liquid, the temperature starts to increase uniformly again.
- Then, there comes a point at which the liquid begins to boil and further heating does not result in an increase in temperature.
The temperature remains constant while the liquid boils.
This constant temperature is known as the boiling point.
- At the boiling point heat is absorbed without any change in temperature (latent heat of vaporization), all the energy absorbed is being used to overcome the intermolecular forces between the particles in the liquid.
- When all the liquid has been vaporized to gas the temperature will once again increase.
Latent heat of fusion (latent enthalpy of melting) results in a constant temperature known as the melting point of the solid.
Latent heat of vaporization (latent enthalpy of vaporisation) results in a constant temperature known as the boiling point of the liquid.
Different pure substances have different melting points and boiling points.
Chemists can use the melting point or boiling point of a pure substance to help identify it.
So, back to the original question of why it is colder at sunrise.
When the sun first rises in the morning it provides heat energy which increases the kinetic energy of molecules in the air which should result in an increase in air temperature, but,
this heat energy is transferred from the air molecules to the water molecules in the "dew" (liquid water) which use it to overcome the intermolecular forces between the water molecules in the liquid, turning the liquid water into gaseous water.
The air surrounding us loses heat energy resulting in a loss of kinetic energy which we observe as a decrease in air temperature.
Cooling Curves and Latent Heat
Let's look at what happens as a pure substance, initially present as a hot gas, is cooled at a constant rate:
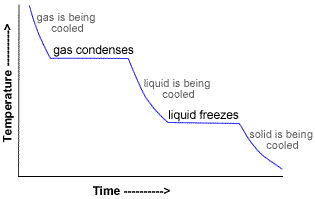
- Initially, the temperature of the gas falls until the boiling point is reached.
- At the boiling point the gas condenses to a liquid, releasing the latent heat of vaporization, causing the temperature to remain constant.
- When all the gas has liquefied or condensed, the temperature falls again until the freezing point is reached.
- At the freezing point the liquid solidifies to a solid, releasing the latent heat of fusion, causing the temperature to remain constant.
- When all the liquid has solidified, the temperature once again decreases.
During distillation, Chemists can identify the substance being vaporised by its boiling point.
Then they condense the hot vapor by cooling it so that it forms a liquid which can be collected in a suitable vessel.
As long as the temperature remains at the same constant temperature, they will be condensing and collecting the same substance.
This is why distillation can be used to obtain a pure substance from a mixture.
Latent Heat and Changes of State
A solid or liquid substance can change state when it is heated:
- Melting or fusion: a solid substance, X(s), absorbs heat energy and melts (undergoes fusion) to form a liquid, X(l).
We can think of energy as being a "reactant" in the chemical equation:
X(s) + energy → X(l)
Reactions that absorb energy are known as endothermic reactions, and the enthalpy change (ΔH) for endothermic reactions has a positive (+) value:
X(s) → X(l) ΔH = + kJ mol-1
The energy absorbed by a solid to change its state to a liquid is known as the latent heat of fusion (latent enthalpy of fusion).
- Vaporisation (vaporization): A liquid substance, X(l) absorbs heat energy to form a gas, X(g).
We can think of energy as being a "reactant" in the chemical equation:
X(l) + energy → X(g)
This is also an endothermic reaction because energy is being absorbed, so the enthalpy change (ΔH) for this change of state has a positive (+) value:
X(l) → X(g) ΔH = + kJ mol-1
The energy absorbed by a liquid to change its state to a gas is known as the latent heat of vaporization (latent enthalpy of vaporisation).
- Sublimation: A solid substance, X(s), absorbs heat energy and forms a gas, X(g), directly without changing into a liquid first.
We can think of energy as being a "reactant" in the chemical equation:
X(s) + energy → X(g)
This is also an endothermic reaction because energy is being absorbed, so the enthalpy change (ΔH) for this change of state has a positive (+) value:
X(s) → X(g) ΔH = + kJ mol-1
The energy absorbed by a solid to change its state to a gas is known as the latent heat of sublimation (latent enthalpy of sublimation).
Solid iodine and solid carbon dioxide (CO2(g)) are two common examples of substances that undergo sublimation when heated.
A liquid or gaseous substance can change state when it is cooled:
- Solidification or freezing: Heat energy is removed from a liquid substance, X(l), in order to form a solid, X(s).
We can think of energy as a "product" of the reaction:
X(l) → energy + X(s)
Chemical reactions that release energy (energy is a "product" of the reaction) are known as exothermic reactions.
For an exothermic reaction, the enthalpy change (ΔH) for the reaction is a negative (-) value:
X(l) → X(s) ΔH = - kJ mol-1
The chemical equation written for solidification or freezing is the reverse of that for melting or fusion.
solidification (freezing): X(l) → energy + X(s)
melting (fusion): X(s) + energy → X(l)
We would expect the value for "energy" for the freezing reaction to be the same as that for melting, but with the opposite sign:
melting (fusion): X(s) → X(l) ΔHfusion = + kJ mol-1
solidification (freezing): X(l) → X(s) ΔHfreezing = -ΔHfusion kJ mol-1
- Condensation or liquefaction: Heat energy is removed from a gaseous substance, X(g), in order to form a liquid, X(l).
We can think of energy as a "product" of the reaction:
X(g) → energy + X(l)
This is an exothermic reaction because energy is produced, so the enthalpy change (ΔH) for the reaction is a negative (-) value:
X(g) → X(l) ΔH = - kJ mol-1
The chemical equation written for condensation or liquefaction is the reverse of that for vaporisation (vaporization).
condensation (liquefaction): X(g) → energy + X(l)
vaporisation (vaporization): X(l) + energy → X(g)
We would expect the value for "energy" for the condensation reaction to be the same as that for vaporisation, but with the opposite sign:
vaporisation (vaporization): X(l) → X(g) ΔHvaporisation = + kJ mol-1
condensation (liquefaction): X(g) → X(l) ΔHcondensation = -ΔHvaporisation kJ mol-1
The table below provides a summary of the energy changes associated with changes state:
| Change of State |
Name |
Energy is |
ΔH = |
ΔH
sign |
|---|
| solid → liquid |
melting
(fusion) |
absorbed
(endothermic) |
Latent Heat of Fusion |
+ |
| liquid → solid |
solidification
(freezing) |
released
(exothermic) |
-Latent Heat of Fusion |
- |
| liquid → gas |
vaporization
(boiling or
evaporation) |
absorbed
(endothermic) |
Latent Heat of Vaporization |
+ |
| gas → liquid |
condensation
(liquefaction) |
released
(exothermic) |
-Latent Heat of Vaporization |
- |
| solid → gas |
sublimation |
absorbed
(endothermic) |
Latent Heat of Sublimation |
+ |
| gas → solid |
condensation |
released
(exothermic) |
-Latent Heat of Sublimation |
- |
Latent Heats for Some Common Substances
Enthalpies of fusion (heat of melting) and enthalpies of vaporisation (heat of evaporation) for some pure substances at the transition temperature for each change of state are given in the table below:
| Melting |
Melting Point
(°C) |
Latent Heat of Fusion
(kJ mol-1) |
Boiling |
Boiling Point
(°C) |
Latent Heat of Vaporization
(kJ mol-1) |
|---|
| H2(s) → H2(l) |
-259 |
0.12 |
H2(l) → H2(g) |
-253 |
0.92 |
| N2(s) → N2(l) |
-210 |
0.36 |
N2(l) → N2(g) |
-196 |
2.8 |
| O2(s) → O2(l) |
-219 |
0.44 |
O2(l) → O2(g) |
-183 |
6.82 |
| CH4(s) → CH4(l) |
-182 |
0.94 |
CH4(l) → CH4(g) |
-161 |
8.18 |
| CH3OH(s) → CH3OH(l) |
-98 |
3.16 |
NH3(l) → NH3(g) |
-33 |
23.35 |
| C2H5OH(s) → C2H5OH(l) |
-114 |
5 |
CH3OH(l) → CH3OH(g) |
64 |
35.27 |
| NH3(s) → NH3(l) |
-78 |
5.65 |
C2H5OH(l) → C2H5OH(g) |
78 |
38 |
| H2O(s) → H2O(l) |
0 |
6.01 |
H2O(l) → H2O(g) |
100 |
40.66 |
The latent heat of fusion for the melting of solid water (ice, H2O(s)) is given in the table as 6.01 kJ mol-1.
This means that 1 mole of solid water (ice) absorbs 6.01 kJ of heat energy at 0°C when it undergoes a change of state to form liquid water.
H2O(s) → H2O(l) ΔHfusion = +6.01 kJ mol-1
The latent heat of fusion for the melting of solid methane (CH4(s)) is given in the table as 0.94 kJ mol-1.
This means that 1 mole of solid methane (CH4(s)) absorbs 0.94 kJ of heat energy at -182°C when it undergoes a change of state to form liquid methane (CH4(l)).
CH4(s) → CH4(l) ΔHfusion = +0.94 kJ mol-1
Note that the latent heat of fusion (latent enthalpy of fusion) for solid hydrogen (H2(s)), nitrogen (N2(s)), oxygen (O2(s)) and methane (CH4(s)) is very low compared to that for methanol (CH3OH(s)), ethanol (C2H5OH(s)), ammonia (NH3(s)) or water (H2O(s)).
Water, ammonia, methanol and ethanol are all polar molecules in which stronger hydrogen bonds act between the molecules making up the solid, while the other substances are non-polar and have only weak intermolecular forces (Van der Waals forces, Dispersion forces, or London Forces) acting between the molecules making up the solid.
Therefore, the energy required to melt water, ammonia, methanol and ethanol is greater than the energy required to melt the other tabulated substances.
The stronger the intermolecular forces acting between the molecules in the solid substance are, the more energy is required to weaken those forces to cause the solid to melt.
That is, in general, the latent heat of fusion (or enthlapy of melting) is greater for polar substances than it is for non-polar substances.
Worked Example of Latent Heat Calculation
Question:
The latent heat of fusion of water is 6.01 kJ mol-1
Determine the quantity of heat in kilojoules required to melt 50 grams of ice at 0°C.
Solution:
(Based on the StoPGoPS approach to problem solving.)
- What is the question asking you to do?
Calculate the heat required to melt solid water at its melting point of 0°C
heat = ? kJ
- What data (information) have you been given in the question?
Extract the data from the question:
ΔHfusion = latent heat of fusion = +6.01 kJ mol-1
m = mass of water = 50 g
T = melting point = constant = 0°C
- What is the relationship between what you know and what you need to find out?
At the melting point the temperature remains constant, all the energy absorbed is weakening intermolecular forces not raising the temperature.
1 mole of solid water (ice) will absorb 6.01 kJ of energy.
(a) Calculate moles of water present :
moles = mass ÷ molar mass
(b) Calculate energy absorbed by this amount of water:
energy absorbed = moles × ΔHfusion
- Perform the calculations
(a) Calculate moles of water present :
moles = mass ÷ molar mass
mass of water = 50 g
molar mass of water (H2O) = 2 × 1.008 + 16.00 = 18.016 g mol-1
(relative atomic mass of H and O from periodic table)
moles of water = 50 g ÷ 18.016 g mol-1 = 2.78 mol
(b) Calculate energy absorbed by this amount of water:
energy absorbed = moles × ΔHfusion
energy absorbed = 2.78 mol × 6.01 kJ mol-1 = 16.7 kJ
- Is your answer plausible?
Work backwards, use your value of heat energy absorbed and the mass of water to calculate the latent heat of fusion:
n(H2O) = m ÷ M = 50 ÷ (2 × 1 + 16) = 2.78 mol
16.7 kJ of energy melted 2.78 mol of water (at constant temperature of 0°C)
Therefore, the energy absorbed per mole = 16.7 kJ ÷ 2.78 mol = 6.01 kJ mol-1
Hence the latent heat of fusion of solid water (ice) is 6.01 kJ mol-1
Since this value agrees with that given in the question, we are reasonably confident that our answer is plausible.
- State your solution to the problem "quantity of heat energy in kJ to melt 50 g of solid water at 0°C":
heat energy absorbed = 16.7 kJ
Footnotes
1. The word "latent" comes from the Latin for "hidden", it is heat energy that we know is being absorbed but it is "hidden" from view because we can't measure it directly as a temperature change.
2. Heat is energy in transit. We say that heat "flows" from hot bodies to cold bodies.
Heat energy can be used to increase the kinetic energy (movement) of particles.
Temperature is a measure of the average of the average kinetic energy of the particles in the system.


