Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Energy Content of Food 2
The human body requires energy in order to undertake proceses like digestion, breathing and moving.
This energy is provided by the food we eat.
The main food groups that provide energy are carbohydrates, proteins and lipids (fats and oils).
The carbohydrates, proteins and lipids (fats and oils) in food are oxidised in our bodies to release energy.
Oxidation of lipids (fats and oils) provides more energy per gram than either proteins or carbohydrates, as shown in the table below:
| Energy Content of Major Food Groups |
|---|
| Nutrient |
Energy
(kJ g-1) 3 |
|---|
| carbohydrates |
17 |
| protein |
17 |
| lipids (fats and oils) |
37 |
The greater the proportion of fat (or oil) in a food, the greater its energy content will be.
Labels on packaged food include a section on the energy content of food.
The energy content of food is often given "per serve of food" and/or "per 100 grams of food".
The energy content on the label of some examples of food you might find in your kitchen is shown in the table below:
| Food |
Energy Content
(kJ/100 g) |
|---|
| breakfast cereal |
1630 |
| sultanas |
1380 |
| cheese |
1250 |
| bread |
1040 |
| baked beans |
383 |
You can add to this table yourself. Just read the labels on your favourite foods and find the values for the energy content.
This table tells us that the energy content of 100 g of this breakfast cereal is 1630 kJ (or 1630 kJ/100 g).
The energy content of 1 g of this breakfast cereal is 1630 kJ ÷ 100 g = 16.30 kJ (or 16.30 kJ g-1 or 16.30 kJ/g)
If I eat 150 g of this cereal for breakfast, it would provide me with 150 g × 16.30 kJ g-1 = 2445 kJ of energy.
My small packet of sultanas also lists an energy content per serving as 552 kJ and a serving size is 40 g.
This means that if I eat 40 g of sultanas they will provide me with 552 kJ of energy.
If I eat 1 g of sultanas they will provide 552 kJ ÷ 40 g = 13.8 kJ (13.8 kJ g-1) of energy.
If I eat 100 g of sultanas they will provide me with 100 × 13.8 = 1380 kJ (13.8 kJ/100 g) of energy.
Because food is a mixture of different substances, its energy content is measured in units of kilojoules per gram of food (kJ g-1).
It is not possible to use units of kilojoules per mole of food (kJ mol-1) because the food we eat is almost exclusively a mixture of different substances.
We can measure the amount of heat energy released when foods are oxidised during a combustion reaction in the school laboratory.
Experiment: Measuring the Heat of Combustion of Food
This experiment can be used to measure the energy content of solid foods that can be easily cut up into small pieces of about 0.5 to 1 cm3.
The food will need to be dried before use, eg, placed in a sealed dessicator with a suitable dessicant for a few days.
Removing moisture from the food will make it easier to burn.
Suggestions of foods to use are
- cheese
- bread
- biscuit
- cake
- pasta
- breakfast cereal
- snack food (chips/crisps, marshmallow, popcorn etc)
| Procedure: 4 |
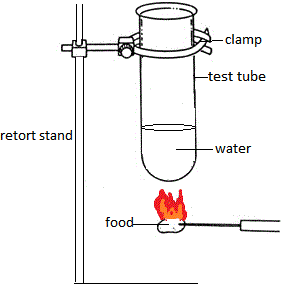
Step 1: Transfer 20.0 mL of water to a test tube using a pipette. 5
Clamp the test tube to a retort stand.
Step 2: Use a thermometer to measure the temperature of the water and record this as the initial temperature (Ti).
Step 3: Use an electronic balance to measure the mass of a small sample of food and record this mass (m).
Step 4: Impale the piece of food on a mounted needle with an insulated handle.
Step 5: Light a bunsen burner at a good distance from the retort stand to prevent heating the water in the test tube and hold the food in the flame until it catches light.
Step 6: As soon as the food is burning, quickly position it under the test tube of water as shown in the diagram on the right.
Step 7: When the food has completely burned away and its flame has extinguished itself, quickly stir the water to ensure that there will be a consistent temperature in the whole body of water, then, measure the temperature of the water in the test tube and record the highest temperature reached as the final temperature (Tf).
|
|
Note: if the flame extinguishes before all the food has combusted, quickly use the bunsen burner to ignite the food again and then reposition the food back under the test tube of water.
Sample Results
The procedure above was used to determine the energy content of a dried biscuit.
The results of this experiment are shown in the table below:
| mass of biscuit |
2.21 g |
| volume of water |
20.00 mL |
| initial water temperature |
19.76 °C |
| final water temperature |
23.39 °C |
Sample Calculations
Calculating the energy content of the food from the results of the experiment is a 3 step process:
- Calculate the amount of heat energy absorbed by the water.
- Calculate the amount of heat energy released by the combustion of the food.
- Calculate the amount of heat energy released per gram of the food combusted.
These calculations are shown below using the results of our experiment given above.
- Determine the amount of heat energy absorbed by the water, qabsorbed:
qabsorbed = mwater × Cg × ΔT
mwater = 20.00 g (assume the density of water is 1.00 g mL-1 then 20.00 mL = 20.00 g)
Cg = specific heat capacity of water = 4.184 J°C-1g-1 (from data sheet)
ΔT = final temperature - initial temperature = 23.39 - 19.76 = 3.63°C
qabsorbed = 20.00 × 4.184 × 3.63 = 303.8 J
- Determine the amount of heat energy released by the combustion of the biscuit sample, qreleased:
Assume no heat is lost, that is, the energy released by the combustion of biscuit all goes towards heating the water, then:
qreleased = qabsorbed = 303.8 J
- Calculate the heat of combustion of the biscuit (enthalpy of combustion of biscuit, or energy content of biscuit):
heat of combustion = qreleased (J) ÷ mass of biscuit (g)
heat of combustion = 303.8 J ÷ 2.21 g = 137.5 J g-1
Results from this experiment will always be less than expected because some heat will be lost to the surroundings.
Note that you could improve on this experimental design by trying to insulate the experiment to prevent heat loss to the surroundings.
Alternatively, you could use a bomb calorimeter, a device designed to minimise errors due to heat loss.
Problem Solving: Worked Example of Enthalpy of Combustion of Food
Question:
The label on a packet of bread gives the energy content of the bread as 1040 kJ per 100 g.
Chris the Chemist has been asked to undertake an experiment to verify this value for the energy content of the bread.
25.00 g of water is placed in a test tube and clamped into place on a retort stand.
Chris carefully weighs a 0.50 g sample of the dried bread.
Chris ignites the bread sample and places it under the water.
Calculate the expected increase in the temperature of the water in °C if the value for the energy content of this bread is accurate and there is no heat loss during the experiment.
Assume the specific heat capacity of water is 4.184 J°C-1g-1
Solution:
(Based on the StoPGoPS approach to problem solving.)
- What is the question asking you to do?
Calculate the increase in water temperature in °C
ΔT = ?°C
- What data (information) have you been given in the question?
Extract the data from the question:
qreleased = energy content of bread = 1040 kJ/100 g
mbread = mass of bread = 0.50 g
mwater = 25.00 g
Cg = specific heat capacity of water = 4.184 J°C-1g-1
- What is the relationship between what you know and what you need to find out?
(i) Note the inconsistency of units. The heat capacity of water is given in J°C-1g-1 but the energy content of bread is given in kJ/100 g.
Convert energy content from kJ/100 g to J g-1
(ii) Use the energy content of bread in J g-1 to calculate how much energy in joules (J) would be released by the combustion of 0.50 g of bread.
qreleased = mbread (g) × energy content (J g-1)
(iii) Assume all the heat energy released by the combustion of the bread goes to heat the water in the test tube (that is, there is no heat lost to the surroundings).
Calculate the amount of energy absorbed by the water
qabsorbed = qreleased
(iv) Calculate the expected temperature increase of the water:
qabsorbed = mwater × Cg × ΔT
qabsorbed ÷ (mwater × Cg) = ΔT
- Perform the calculations
(i) Note the inconsistency of units. The heat capacity of water is given in J°C-1g-1 but the energy content of bread is given in kJ/100 g.
Convert energy content from kJ/100 g to J g-1
energy content of 100 g of bread = 1040 kJ
divide energy content by 100 to find energy content in kJ g-1
energy content = 1040 kJ ÷ 100 g = 10.40 kJ g-1
multiply this energy content by 1000 to find the energy content in J g-1
energy content = 10.40 kJ g-1 × 1000 J kJ-1 = 10 400 J g-1
(ii) Use the energy content in of bread in J g-1 to calculate how much energy in joules (J) would be released by the combustion of 0.50 g of bread.
Calculate the energy released by combustion of 0.50 g of bread:
qreleased = mbread (g) × energy content (J g-1)
qreleased = 0.50 g × 10 400 J g-1 = 5 200 J
(iii) Assume all the heat energy released by the combustion of the bread goes to heat the water in the test tube (that is, there is no heat lost to the surroundings).
Calculate the amount of energy absorbed by the water
qabsorbed = qreleased = 5 200 J
(iv) Calculate the expected temperature increase of the water:
qabsorbed = mwater × Cg × ΔT
5 200 J = 25.00 g × 4.184 J°C-1g-1 × ΔT °C
5 200 = 104.6 × ΔT
ΔT = 5 200 ÷ 104.6 = 49.7°C
- Is your answer plausible?
Work backwards using your calculated value for the increase in water temperature to calculate the energy content of the bread.
Calculate heat absorbed by water:
qabsorbed = mwater × Cg × ΔT = 25 × 4.184 × 49.7 = 5 200 J = 5 200 J ÷ 1000 J/kJ = 5.20 kJ
Heat absorbed by water = heat released by combustion of 0.50 g of bread = 5.20 kJ
heat released per gram of bread = 5.20 kJ ÷ 0.5 g = 10.4 kJ
heat released by 100 g of bread = 10.4 kJ × 100 = 1040 kJ
energy content of bread is 1040 kJ per 100 g
Since this value for the energy content is the same as that given in the question (1040 kJ/100 g) we are reasonably confident that our answer for the temperature increase is plausible.
- State your solution to the problem "expected temperature increase of water in °C":
ΔT = 49.7 °C
Footnotes
1. The joule is named after the English physicist James Prescott Joule.
The Ninth International Conference on Weights and Measures (1948) recommended the use of the joule (volt coulomb) as the unit of heat.
The joule is a derived SI unit for the measurement of energy.
The SI base unit for the measurement of energy is kg.m2 s-2
1 J = 1 kg.m2 s-2
There lots of units for energy in common use. You will find a tutorial on converting between joules (kilojoules etc) and calories here.
1 calorie (cal) = 4.1840 J
1 kilocalorie (kcal) = 4184 kJ
2. Heat, or heat energy, is the energy directly transferred from one object to another.
Heat is energy in transit, a substance like food or water at a constant temperature does not have a "heat content", but it does have an "energy content".
The energy content of a substance is made up of the kinetic energy (movement) of its particles and potential energy such as the stored chemical potential energy in its chemical bonds.
Temperature is a measure of the average kinetic energy of the particles.
3. The table below gives the energy content of the major food groups in kilocalories per gram:
| nutrient |
energy content
(kcal g-1) |
|---|
| carbohydrate |
4 |
| protein |
4 |
| lipid (fat or oil) |
9 |
4. Your experiment might look a bit different to this, for example:
(a) You might impale your food on a needle mounted on a cork positioned under the water.
(b) You might enclose your whole experiment in a foil-lined box to try to prevent heat loss
While these measures may affect the results of your experiment (that is, they may affect the temperature increase of the water) they will not affect HOW you calculate the energy content of the food.
5. Alternatively you could weigh the water and obtain its mass directly.

