Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Identifying Equilibrium in Chemical Reactions
For the chemical reaction in which reactants combine to produce products:
The forward reaction is: reactants → products
The reverse reaction is: products → reactants
Equilibrium reaction is: reactants ⇋ products
The changes in the concentration of reactants and products in a closed reaction vessel were measured over time.
The results of the experiment are shown below in the table and plotted on the graph:
| Time (h) |
[reactants](mol L-1) |
[products](mol L-1) |
|---|
| 0 |
0.40 |
0 |
| 2 |
0.22 |
0.22 |
| 4 |
0.08 |
0.28 |
| 6 |
0.06 |
0.30 |
| 8 |
0.06 |
0.30 |
| 10 |
0.06 |
0.30 |
|
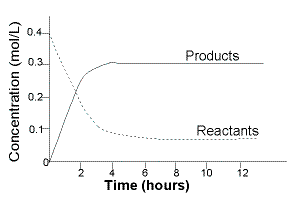 |
Initially, at time=0, only reactants are present in the reaction vessel.
Concentation of reactants is at their maximum value.
Concentration of products is 0, [products] = 0
After time 0, reactants begin to react to produce products.
The concentration of reactants decreases with time.
The concentration of products increases with time.
A little after 4 hours:
The concentration of reactants remains steady at 0.06 mol L-1.
The concentration of products remains steady at 0.30 mol L-1.
Equilibrium is reached a little after 4 hours.
At equilibrium
the rate of the forward reaction = the rate of the reverse reaction
That is, the rate at which reactants are combining to produce products is exactly the same as the rate at which products are breaking apart to re-produce reactants.
So at equilibrium the concentration of reactants remains constant and the concentration of products remains constant.
Equilibrium has been reached when:
- concentration of reactants over time does not change
and
- concentration of products over time does not change
Affect of a Catalyst
Because some chemical reactions occur slowly, industrial processes often use a catalyst to speed up the reaction.
A catalyst speeds up the rate of both the forward and reverse reactions, that is,
- Rate of forward reaction increases.
That is, the rate at which reactants disappear increases.
- Rate of reverse reaction increases.
That is, the rate at which products appear increases.
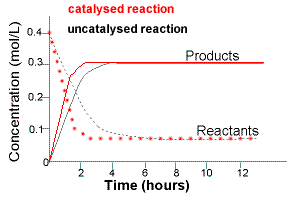
The graph below shows how the concentration of reactants and products in the SAME reaction at the same temperature and pressure changes over time in two different situations:
(i) No catalyst is used during the reaction
(uncatalysed reaction)
(ii) A catalyst is used during the reaction
(catalysed reaction)
Note that the initial slope of the concentration vs time lines will be steeper for both reactants and products in the catalysed reaction.
For the catalysed reaction:
(a) reactants disappear faster
(b) products are formed faster.
|
|
A catalyst does NOT affect the equilibrium position.
At equilibrium the concentrations of reactants and products for the catalysed reaction are the same as for the uncatalysed reaction.
A catalyst ONLY changes the time taken for the reaction to reach equilibrium.
Affect of a Temperature Change on Equilibrium
Some chemical reactions occur slowly at room temperature, but by heating the reaction mixture we can speed up the rate of the reaction.
Increasing the temperature of a reaction mixture speeds up the rate of both the forward and reverse reactions.
If temperature is increased:
- Rate of forward reaction increases.
That is, the rate at which reactants disappear increases.
- Rate of reverse reaction increases.
That is, the rate at which products appear increases.
However, the final equilibrium position, that is, the concentration of reactants and of products once equilibrium is reached, is determined by whether the reaction is exothermic or endothermic (see Le Chatelier's Principle).
In an exothermic reaction, heat is a product.
reactants ⇋ energy + products
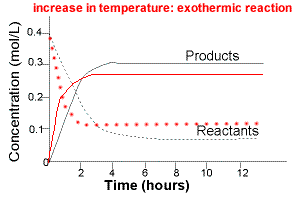
The graph below compares the concentration of reactants and products over time for the same exothermic reaction under two different sets of conditions:
(i) lower temperature
(ii) higher temperature
The intial rate of formation of products is greater at higher temperatures.
The intial rate of disappearance of reactants is greater at higher temperatures.
The equilibrium concentration of reactants will be higher at higher temperature.
The equilibrium concentration of products will be lower at higher temperature.
|
|
Increasing the temperature of an exothermic reaction favours the reverse reaction, that is, increasing temperature favours the formation of reactants.
| |
For an Exothermic Reaction |
|---|
| reaction conditions |
initial rate
forward reaction |
initial rate
reverse reaction |
equilibrium concentration
of reactants |
equilibrium concentration
of products |
|---|
| low temperature |
slower |
slower |
lower |
higher |
|---|
| high temperature |
faster |
faster |
higher |
lower |
|---|
In an endothermic reaction, heat is a reactant.
reactants + energy ⇋ products
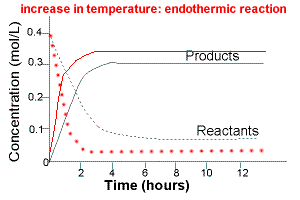
The graph below compares the concentration of reactants and products over time for the same endothermic reaction under two different sets of conditions:
(i) lower temperature
(ii) higher temperature
The intial rate of formation of products is higher at higher temperatures.
The intial rate of disappearance of reactants is higher at higher temperatures.
The equilibrium concentration of products will be higher at higher temperature.
The equilibrium concentration of reactants at higher temperature will be lower.
|
|
Increasing the temperature of an endothermic reaction favours the formation products.
| |
For an Endothermic Reaction |
|---|
| reaction conditions |
initial rate
forward reaction |
initial rate
reverse reaction |
equilibrium concentration
of reactants |
equilibrium concentration
of products |
|---|
| low temperature |
slower |
slower |
higher |
lower |
|---|
| high temperature |
faster |
faster |
lower |
higher |
|---|
Disturbing the Equilibrium
Once a system has reached equilibrium the concentration of reactants and products remains constant (this is how chemical equilibrium is defined!).
But it is possible to disturb (or perturb) the system so that the rate of either the forward or reverse reaction is temporarily increased.
This can be done by making a change to the conditions of the reaction by:
- changing the temperature
- changing the concentrations
- changing the volume (for gases)
- changing the pressure (for gases)
Overtime, the system will adjust to the change and equilibrium will be re-established, BUT, the equilibrium concentration of reactants and products will have been changed.
Le Chatelier's Principle is used to predict the new equilibrium position.
Example: Affect of a Change in Concentration on a System at Equilibrium
A system at equilibrium can be represented by the chemical equation below:
reactants ⇋ products
If this system is allowed to come to equilibrium and then more reactants are added, ie, increasing the concentration of reactants, then
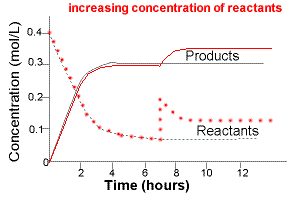
- at the time reactants are added there would be an instantaneous increase in reactant concentration
- followed by a readjustment as more reactants react to produce products according to Le Chatelier's Principle
- A new equilibrium position is established in which the concentration of reactants added and products produced is higher than before.





