Electrolysis of Molten, or Liquid, Salts Chemistry Tutorial
Key Concepts
- Melting a salt enables it to conduct electricity.
non-conductor → conductor MX(s) heat
→M+(l) + X-(l) - The use of inert electrodes, electrodes made of a material that will not take part in the reactions, means the only species present that can take part in the electrolytic cell reactions are the anions and cations of which the salt is composed.
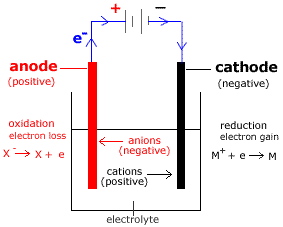
- During the electrolysis of a molten salt:
(a) Oxidation occurs at the anode (positive electrode).
(Oxidation, loss of electrons, occurs at the anode)(b) Anions (negatively charged ions) migrate to the positive anode (anode).
(c) Reduction occurs at the cathode (negative electrode).
(Reduction, gain of electrons, occurs at the cathode)(d) Cations (positively charged ions) migrate to the negative cathode (cathode).
- Reactions occurring during electrolysis of a molten, or liquid, salt (MX(l)) are:
oxidation at anode
(positive electrode)X-(l) → X + e- reduction at cathode
(negative electrode)e- + M+(l) → M - Electrolysis is a non-spontaneous reaction:
Ecell for the electrolytic cell is negative1.
- Applied emf must be greater than the emf for the cell, ie greater than -Ecell.
- Mass of substance produced electrolytically is proportional to the quantity of electricity flowing.