Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
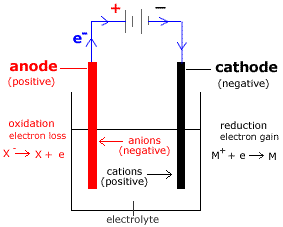
Electrolytic Cell
A supply of electricity such as a battery or a power pack in the school laboratory is required to drive the electrolytic cell reactions.
Consider the electrolytic cell shown below for the electrolysis of MX:
- The electrolyte contains MX (made up of M+ cations and X- anions).
- Anode is positive (+).
Oxidation (loss of electrons) occurs at the anode.
- Cathode is negative (-).
Reduction (gain of electrons) occurs at the cathode.
- Anions (X-) flow towards the anode (positive electrode).
- Cations (M+) flow towards the cathode (negative electrode).
- Electrons flow from the anode (+) to the cathode (-) through the wires connecting the electrodes to the power supply.
The reactions occurring in this electrolytic cell can be represented as shown below:
| Electrolytic Cell Reactions |
|---|
anode:
(oxidation) |
X- |
→ |
X + e- |
Eo1 |
cathode:
(reduction) |
M+ + e- |
→ |
M |
Eo2 |
| |
|
| cell: |
X- + M+ |
→ |
X + M |
Eocell = -ve |
Note: Eocell = Eo1 + Eo1 = negative
EMF (volts) required to drive this non-spontaneous reaction is greater than Eocell for the spontaneous redox reaction.
Comparison of Galvanic (Voltaic) Cells and Electrolytic Cells
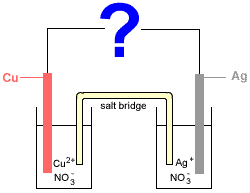
Consider the following electrochemical cell made up of two half-cells connected by a salt bridge:
- Half-Cell 1: a solid copper electrode (Cu) sits in an aqueous solution of copper(II) nitrate (Cu(NO3)2(aq))
- Half-Cell 2: a solid silver electrode (Ag) sits in an aqueous solution of silver nitrate (AgNO3(aq))
The relevant half-cell equations obtained from a table of standard reduction potentials are:
| Cu2+ + 2e- → Cu(s) |
Eo = +0.34 V |
| Ag+ + e- → Ag(s) |
Eo = +0.80 V |
Whether copper (Cu) is oxidised to copper ions (Cu2+), or, copper ions (Cu2+) are reduced to copper (Cu) depends on what the ? in the diagram is.
If ? = voltmeter, galvanometer, or metal wire
If we replace the ? in the diagram with a voltmeter or galvanometer to complete the circuit the reaction would proceed spontaneously:
anode
(oxidation): |
Cu(s) |
→ |
Cu2+ + 2e- |
Eo = -0.34V |
cathode
(reduction): |
2Ag+ + 2e- |
→ |
2Ag(s) |
Eo = +0.80V |
| |
|
cell:
(redox) |
Cu(s) + 2Ag+ |
→ |
Cu2+ + 2Ag(s) |
Eo = -0.34 + 0.80
Eo= +0.46 V |
Electrons would spontaneously flow from the negative copper anode to the positive silver cathode generating electricity which would be measured by the voltmeter or galvanometer.
The copper anode would disintegrate and silver would be deposited on the silver cathode.
This type of electrochemical cell is known as a galvanic or voltaic cell.
If ? = battery or other electricity supply with emf > 0.46 V
If we replace the ? in the diagram with a battery supplying more than 0.46 V then the battery has greater electron pushing power than the spontaneous reaction in the electrochemical cell, forcing the reactions to go in the opposite direction to those above.
cathode
(reduction): |
Cu2+ + 2e- |
→ |
Cu(s) |
Eo = +0.34 V |
anode
(oxidation): |
2Ag(s) |
→ |
2Ag+ + 2e- |
Eo = -0.80 V |
| |
|
cell:
(redox) |
Cu2+ + 2Ag(s) |
→ |
Cu(s) + 2Ag+ |
Eo = +0.34 + -0.80
Eo = -0.46 V |
Electrons are pulled out of the positive silver anode and flow to the negative copper cathode.
This reaction is not generating electricity it is using electricity!
The silver anode disintengrates while copper deposits on the copper cathode.
This type of electrochemical cell is known as an electrolytic cell.