Electrowinning Copper Chemistry Tutorial
Key Concepts
- Electrowinning refers to the process of using electrolysis to extract a metallic element from the compounds in its ore.
- Copper occurs on Earth both as native copper (the uncombined element, Cu), and in ores (copper compounds, or mixtures of compounds, from which copper metal can be extracted profitably).
- Copper ores are usually copper oxides, copper sulfides or copper carbonates.
- Most copper oxide ores do not contain a high enough percentage of copper to make direct smelting of the ore economically viable.
- Electrowinning can be used to extract the copper from these copper oxide ores.
- The process for extracting copper from copper oxide ores can be summarized as:
- Mining : digging or blasting to obtain the ore from the surrounding rock.
- Crushing : mined ore is crushed into very small pieces.
- Grinding : crushed ore is ground into a powder.
- Concentrating : adding water to the powder to create a slurry containing about 15% copper.
- Leaching : sulfuric acid is added to the concentrated slurry to dissolve the copper producing an acidified aqueous solution of Cu2+.
Sulfuric acid is an oxidizing agent (oxidant) but not a very strong one unless it is hot and concentrated.
Copper metal is a reducing agent (reductant) but not a very stong one.
Copper metal won't dissolve in nonoxidizing acids like HCl, but will dissolve in hot concentrated H2SO4:
Cu(s) + 3H2SO4 → Cu2+ + SO2(g) + 2HSO4- + 2H2O
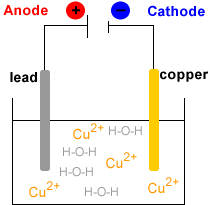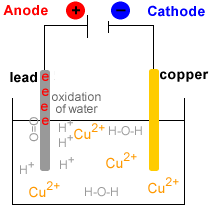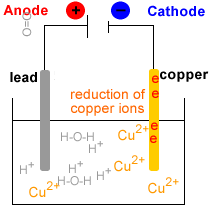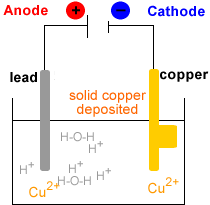
- Electrowinning : electrolysis of the Cu2+(aq) solution to produce metallic copper, Cu(s).