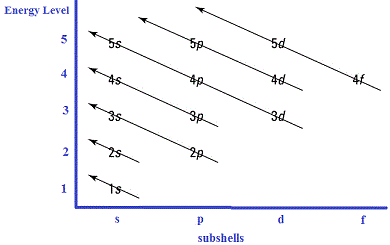
1Experimental evidence reveals that for the transition metals, the 3d subshells are actually of lower energy than the 4s subshell.
Hence, period 4 transition metals ionise by losing the electrons in the highest energy levels first, that is, from the 4s subshell.
These 4s electrons are shielded by the lower energy 3d electrons, making them easier to remove.
For the transition metals it is energetically favourable for electrons to occupy both the 4s and 3d subshells rather than just filling the lower energy 3d subshell first.
The graphical representations of "filling order" are therefore only a general guide to writing an electronic configuration, in reality the actual energy values for subshells are different for each atom based on considerations of electrostaic-type attractions and repulsions between the species making up each atom, so that these general representations should be "tweaked" to represent the energy values for each particular atom.
2This is arrived at by the application of "Hund's Rule": if multiple orbitals of the same energy level are available, electrons fill unoccupied orbitals singly first before pairing up with an electron in the same orbital.
3Notice that writing the electronic configuration of the transition metals in this way, ie, 3d before 4s, actually gives a better representation of the relative energies of the 3d and 4s electrons, that is, 4s electrons are of higher energy than 3d electrons and hence are written last in the electronic conifguration.