Downs Cell for the Production of Sodium Chemistry Tutorial
Key Concepts
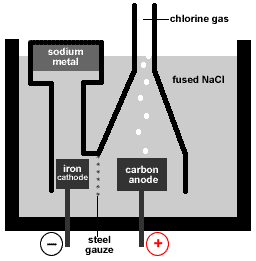
- Sodium metal can be extracted electrolytically from molten (fused) sodium chloride using a Downs Cell.
- Sodium chloride has a high melting point, ~800oC, so calcium chloride or sodium carbonate is added to reduce the melting point to around 600oC.
- The products of the electrolysis of molten (fused) sodium chloride are sodium metal and chlorine gas.
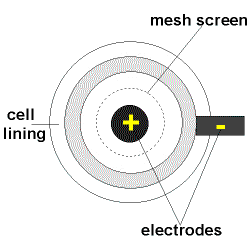
- In the Downs Cell a circular iron cathode surrounds the carbon anode and the products of the electrolysis are separated by steel mesh or gauze to prevent them coming into contact and reforming sodium chloride.
- The amount of sodium produced can be calculated using Faraday's Laws of Electrolysis.