- What is the question asking you to do?
Predict what will be observed as this experiment proceeds.
- What data (information) have you been given in the question?
Extract the data from the question:
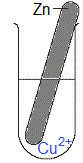
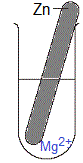
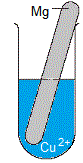
Solid metal is magnesium, Mg(s)
Solution is blue aqueous copper(II) sulfate, CuSO4(aq)
Table of Standard Reduction Potentials (data sheet)
- What is the relationship between what you know and what you need to find out?
(i) When copper(II) sulfate dissolves in water to form an aqueous solution, copper(II) ions (Cu2+(aq)) and sulfate ions (SO42-(aq)) will be present in the solution.
(ii) If magnesium metal is a stronger reductant than copper metal, then:
(a) magnesium atoms will be oxidised to magnesium ions
Mg(s) → Mg2+(aq) + 2e-
(b) and copper ions will be reduced to copper atoms
Cu2+(aq) + 2e- → Cu(s)
(iii) If magnesium metal is a weaker reductant than copper metal, no displacement reaction will occur.
(iv) Refer to the Table of Standard Reduction Potentials.
Magnesium is a stronger reductant than copper so magnesium atoms will be oxidised and copper ions will be reduced.
- Decide what observations could be made about the reaction.
(i) The piece of magnesium metal disintegrates, it is visibly smaller in size as the experiment proceeds.
(magnesium ions are being formed and these enter the solution)
(ii) Solid copper deposits in the test-tube.
Solid copper will probably look red-gold or red-brown in colour.
Because the solid copper is more dense than water, it will usually fall to the bottom of the test tube.
(iii) The blue colour of the copper(II) sulfate solution will fade, that is, over time the solution will be less blue in colour.
As the copper(II) ions are consumed in the production of copper atoms, the concentration of copper(II) ions decreases which results in a decrease in the intensity of the blue colour in the test tube.
- Is your answer plausible?
Use some logic based on your understanding of the relative reactivity of magnesium and copper metals.
Magnesium is a Group 2 metal that you should know is quite reactive.
A metal is reactive if it loses electrons easily, that is, if it can be easily oxidised.
A metal that is easily oxidised is a strong reductant.
One of the reasons you do not see household objects made of magnesium is because it is reactive, BUT, it is used in things like fire-starters because it readily reacts.
Copper, on the other hand, is not a very reactive (active) metal.
People in ancient times used copper to make household goods because the metal can be found in its native (unreacted) state.
Even today you will still find copper being used to make household items.
Clearly then, copper metal is not so easily oxidised, that is, it is not as active as magnesium, and therefore copper is a weaker reductant than magnesium.
Given the opportunity, magnesium atoms will oxidise to magnesium ions, and copper ions will reduce to copper atoms.
So, our observations about the mass of magnesium atoms decreasing, the concentration of copper ions decreasing, and the deposition of solid copper, is looking plausible.
- State your solution to the problem:
Observations:
(i) The piece of magnesium becomes smaller in size over time.
(ii) A new red-brown solid is seen in the test tube.
(iii) The blue colour of the solution fades over time.
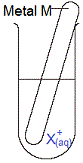 A piece of metal M(s) is placed in an aqueous solution of metal X ions, X+(aq).
A piece of metal M(s) is placed in an aqueous solution of metal X ions, X+(aq).