Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Physical Properties of Covalent Network Solids
The elements carbon, silicon and boron form covalent networks instead of covalent molecules.
Silicon dioxide, SiO2, also exists as a covalent network and is known as quartz or silica.
Its structure is similar to diamond (see table below).
In general, covalent network solids:
⚛ have high melting points
⚛ do not conduct heat or electricity well, they are insulators (graphite, see below, is an exception)
⚛ are hard (graphite, see below, is an exception)
Examples of Covalent Networks: Carbon
Carbon forms 2 naturally occurring covalent network solids:
The difference in the arrangement of the atoms in these two allotropes of carbon leads to differences in their physical properties as shown in the table below:
| |
allotropes of carbon |
|---|
| graphite |
diamond |
| Structure |
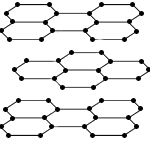 |
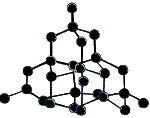 |
|---|
| Bonding |
Each carbon atom makes 3 covalent bonds |
Each carbon atom makes 4 covalent bonds |
|---|
| Description of Structure |
Carbon atoms in a hexagonal arrangement forming layers |
Carbon atoms in a tetrahedral arrangement |
|---|
| Melting Point |
Very high (sublimes at ≈ 3500K) due to the large amount of energy required to break strong covalent bonds |
Very high (≈ 4000K) due to the large amount of energy required to break strong covalent bonds |
|---|
| Electrical Conductivity |
Good conductor between the carbon layers due to delocalised electrons between the carbon layers |
Insulator due to no delocalised electrons (all electrons are used in covalent bonding) |
|---|
| Hardness |
Soft (1-2 on Mohs scale) because the carbon layers can slide over each other |
Hardest known natural mineral (10 on Mohs scale) |
|---|
| Colour |
Black |
Colourless |
|---|
| Uses |
Lubricant due to its softness |
Abrasive due to its hardness |
|---|
When a network covalent solid is heated, a lot of energy needs to be supplied to weaken the strong covalent bonds between the atoms in the 3-dimensional lattice so that they can move over each other, that is, a lot of energy needs to be supplied to melt the solid.
In general, because the atoms and their electrons are bound up in the 3-dimensional structure there are no mobile ions or electrons to carry a charge (or heat), so 3-dimensional covalent network solids are not good conductors of heat or electricity, they are insulators (graphite is an exception because of its layered structure).
In general, because the atoms are held together in the lattice by strong covalent bonds, the structure itself is hard (graphite is an exception because of its layered structure).
Physical Properties of Covalent Molecular Substances
When two atoms share electrons a covalent bond is formed between them.
When the system does not extend infintely in 2 or 3 dimensions, that is, there are a countable number of atoms covalently bonded together in a discrete unit, we use the term "molecule" to refer to this discrete unit.
A molecular substance, or covalent molecule substance, consists of discrete molecules, that is, the system of atoms and covalent bonds does NOT extend infinitely in 3 dimensions as it does in a covalent network.
It is sometimes useful to think of a small molecule as a ball.
There are many examples of molecular, or covalent molecular, substances:
- Elements that exist as covalent molecular substances at room temperature and pressure, examples include:
hydrogen gas, H2(g)
oxygen gas, O2(g)
nitrogen gas, N2(g)
fluorine gas, F2(g)
chlorine gas, Cl2(g)
- Non-metallic compounds that exist as covalent molecular substances at room temperature and pressure, examples include
liquid water, H2O(l)
carbon monoxide gas, CO(g)
carbon dioxide gas, CO2(g)
nitrogen dioxide gas, NO2(g)
ammonia gas, NH3(g)
Consider the melting points of some of these covalent molecular substances as shown in the table below:
| Covalent Molecular Substance |
Melting Point (°C) |
|---|
| hydrogen (H2) |
-259 |
| oxygen (O2) |
-219 |
| nitrogen (N2) |
-210 |
| water (H2O) |
0 |
The melting points are all low, much, much lower than the thousands of degrees needed to melt the covalent network solids of graphite or diamond.
This is because the forces of attraction acting between discrete molecules are much, much weaker than the strong covalent bonds holding covalent network solids together.
These "forces of attraction" are referred to as intermolecular forces.
Note that solid lines are used to represent the covalent bonds holding atoms together in a molecule, for example H2 is represented as H-H
Dotted lines are used to represent the much weaker intermolecular forces acting between discrete molecules, for example the weak intermolecular forces acting between molecule of H2 could be represented as, H-H....H-H
It requires comparatively little energy to weaken these already weak interactions between the discrete molecules in order to melt a covalent molecular substance.
Discrete covalent molecular substances are electrically neutral so they will not conduct electricity when they exist as solids or liquids.


