Corrosion Chemistry Tutorial
Key Concepts
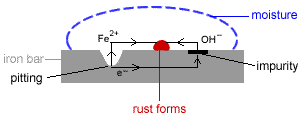
- Corrosion: the oxidation of metals by certain substances such as water and oxygen in the environment.
- Rusting of iron is a common form of corrosion.
- Noble Metal: a metal resistant to corrosion and found uncombined in nature, eg, gold and silver.
Noble metals are also known as Cathodic Metals, or Protected Metals.
- Ignoble Metal: a metal that corrodes.
Ignoble metals are also known as Anodic Metals, or Corroding Metals.
- Passivating metal: a reactive metal that forms an inactive coating as a result of reacting with substances such as oxygen and water, eg, aluminium and chromium.
- In general, corrosion is a spontaneous electrochemical process1 which requires:
(i) an anode where oxidation occurs
(ii) a cathode where reduction occurs
(iii) a metal path to allow for the flow of electrons
(iv) an electrolyte
- Common methods of corrosion prevention are:
(i) Painting
(ii) Plating with another metal
(iii) Cathodic protection
(iv) Alloying with another metal