Sodium Hydroxide and Chlorine Production by Electrolysis
Key Concepts
- Sodium hydroxide, NaOH, is also known as lye or caustic soda.
- Sodium hydroxide is a commonly used base.
- Electrolysis of concentrated sodium chloride solutions (brine) produces chlorine gas, hydrogen gas and aqueous sodium hydroxide.
2NaCl(aq) + 2H2O(l) → H2(g) + Cl2(g) + 2NaOH(aq)
- Cl2(g) is produced at the anode (positive electrode).
- H2(g) and NaOH(aq) are produced at the cathode (negative electrode).
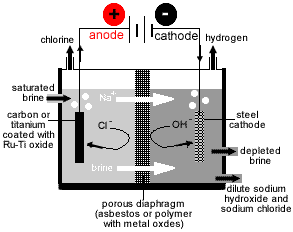
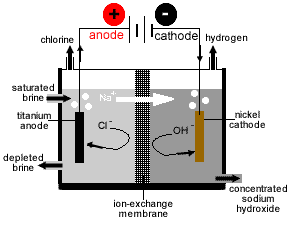
- Three types of electrolytic cell are used to produce sodium hydroxide from brine:
- Castner-Kellner Cell (Mercury Process)
- Nelson Diaphragm Cell
- Membrane Cell