Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Mirror Image Molecules and Chirality
Your left and right hand are chiral. Your hands display the property of chirality.4
Your left hand is the mirror image of your right hand, but, you cannot superimpose your left hand onto your right hand.
Many biological molecules like amino acids, proteins and sugars are chiral, just like your left and right hand, but, only one of these chiral molecules might display biological activity.5
Just like your left hand doesn't fit into the right-handed glove, a "left-handed" protein won't fit into a "right-handed" enzyme, so the rate of the chemical reaction for the "left-handed" protein will not be affected by the "right-handed" enzyme.
Consider a molecule of methane, CH4.
We could use a molecular model kit to build a 3-dimensional model of a molecule of methane, as shown below6:
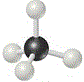
The central carbon atom is the black ball in the centre, each grey ball represents a hydrogen atom.
Two of the C-H bonds lie in the same plane (coloured red below).
One C-H bond extends forward, towards you as you read this (coloured blue below).
One C-H bond extends backwards, away from you as you read this (coloured green below).
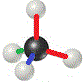
Chemists don't usually use colours to show the 3-dimensional structure of a molecule, instead we draw different kinds of lines and shapes to represent how the atoms are positioned in space.
When we do this, we refer to the representation as a 3-dimensional structural formula:
- Bonds in the same plane (the red lines above) are shown as a solid line
- Bonds that extend towards you (the blue line above) are shown as a solid wedge
- Bonds that extend away from you (the green line above) are shown as a dashed wedge
Ball and Stick
Molecular Model |
|
3-dimensional
structural formula |
|---|
 |
≡ |
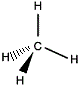 |
Imagine you can take a molecule of methane and place it in front of a mirror.
In the mirror image, the central carbon atom will appear the same distance "behind" the mirror as the central carbon atom of the "real" molecule is in front of the mirror.
The mirror image version of each hydrogen atom will seem to be as far "behind" the mirror as the real version in front of the mirror.
| "real methane" |
mirror |
"mirror image methane" |
|---|
 |
|
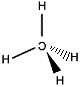 |
We've written the mirror images of "C" and "H" to highlight which is the "real" molecule and which is the "mirror image" molecule but clearly both carbon atoms are the same as each other, and all the hydrogen atoms are indistinguishable from each other, it is really only the arrangement of these atoms in 3-dimensional space that is important to the following discussion.
The two methane molecules, "real" and "mirror image" are the same.
We can rotate the "mirror image" molecule around the vertical axis to produce the same arrangement of C-H bonds in 3-dimensions as the "real" molecule.
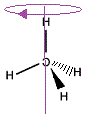 |
→ |
 |
We can see that the "mirror image" methane molecule can be superimposed on the "real" methane molecule by simply rotating the molecule about the vertical axis.
Methane, CH4, is NOT an example of a chiral molecule. We say that methane is achiral.
What if another atom, for example a chlorine atom, is substituted for one of the hydrogen atoms.
Will chloromethane, CH3Cl, and its mirror image, have the same arrangement of atoms in 3 dimensions or different?
Will chloromethane, CH3Cl, be chiral or achiral?
| "real chloromethane" |
mirror |
"mirror image chloromethane" |
|---|
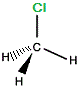 |
|
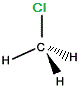 |
If we rotate "mirror image" chloromethane around the vertical axis we will arrive at exactly the same 3-dimensional arrangement of atoms as in the "real" chloromethane molecule.
The "mirror image" chloromethane molecule is superimposable on the "real" chloromethane molecule.
Chloromethane is not chiral, it is said to be achiral.
What if one of the hydrogen atoms in chloromethane is substituted for a different atom, an atom of bromine for example.
Will bromochloromethane and its mirror image have the same arrangement of atoms in 3-dimensions or different?
Will bromochloromethane be chiral or achiral?
| "real bromochloromethane" |
mirror |
"mirror image bromochloromethane" |
|---|
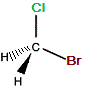 |
|
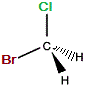 |
The C-Cl bond and the C-Br bond were represented as being in the same plane on the "real" molecule.
On the mirror image, we could rotate the molecule until the bromine atom is "behind" the plane (dashed wedge), then choose to represent the molecule so that both the C-Cl and C-Br bonds are in the same plane, so that one C-H bond would extend towards us and the other C-H extend away from us, so these two representations, "real" and "mirror image" are the same, so bromochloromethane is NOT chiral, it is achiral.
What if we replace one of the remaining hydrogen atoms in bromochloromethane with a different atom, fluorine for example.
Will bromochlorofluoromethane and its mirror image have the same 3 dimensional arrangement of atoms, or will the arrangement of atoms be different?
Is bromochlorofluoromethane chiral or achiral?
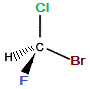
| "real bromochlorofluoromethane" |
mirror |
"mirror image bromochlorofluoromethane" |
|---|
 |
|
 |
If we rotate the "mirror image" molecule so that C-Br bond is "behind" (dashed wedge), then the C-F bond will be in the same plane as the C-Cl bond and the C-H bond will be "forward" (solid wedge), as shown below:
 |
rotate
→ |
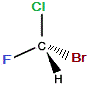 |
If we now represent this rotated "mirror image" molecule with the C-Br bond in the same plane as the C-Cl bond (as it was in the "real" molecule), we now find the C-F bond extends away from us (dashed wedge), and the C-H bond is towards us (solid wedge), as shown below:
 |
≡ |
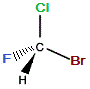 |
Now compare the "real" molecule and this rotated version of the "mirror image" molecule side-by-side:
| "real" molecule |
|
rotated "mirror image" molecule |
|---|
 |
≢ |
 |
When we try to superimpose the "mirror image" molecule onto the "real" molecule we find that the C-Cl and C-Br bonds superimpose, but, the C-F bond in the "real" molecule extends out of the plane towards you while in the "mirror image" molecule it extends behind the plane and away from you.
Similarly, the C-H bond in the "real" molecule extends behind the plane and away from you, but in the "mirror image" molecule the C-H bond extends out of the plane and towards you.
We cannot superimpose the "mirror image" bromochlorofluoromethane molecule onto the "real" molecule.
Bromochlorofluoromethane IS a chiral molecule.
The carbon atom that has 4 different substituents is referred to as chiral centre (sometimes as a chiral carbon).
The carbon atom in a molecule of bromochlorofluoromethane is a chiral centre because it has 4 different substituents (H, Br, Cl and F).
These two molecules, the "real" and "mirror image" bromochlorofluoromethane, are referred to as enantiomers.
Identifying Chiral Centres
In an organic molecule, any carbon atom with 4 different substituents will be a chiral centre.
An organic molecule with a chiral centre will be a chiral molecule.7
Consider the following molecule:
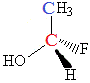
It contains 2 carbon atoms, one is shown as blue, the other as red.
Attached to the blue carbon atom, C, are 3 hydrogen atoms and 1 carbon atom.
Since there are only 2 different substituents (H and C) attached to this carbon atom, this carbon atom is NOT a chiral centre.
Now consider the red carbon atom, C.
Attached to this carbon atom is one OH group, one H atom, one F atom and one methyl group (CH3).
There are 4 different substituents on this carbon atom.
This carbon atom is a chiral centre.
This molecule will be chiral.
Consider the molecule 2-bromobutane shown below with carbon atoms numbered 1, 2, 3 and 4:
| |
H
| |
|
Br
| |
|
H
| |
|
H
| |
|
| H- |
C1 |
- |
C2 |
- |
C3 |
- |
C4 |
-H |
| |
|
H |
|
|
H |
|
|
H |
|
|
H |
|
It is clear that carbons 1 and 4 cannot be chiral centres because they have 3 hydrogen atoms bonded to them so they do not have 4 different substituents.
Similarly carbon 3 cannot be a chiral centre because it has 2 hydrogen atoms bonded to it so it does not have 4 different substituents and cannot therefore be a chiral centre.
But what about carbon 2? Is it a chiral centre?
Let's redraw the structure to make it clearer...
Now we can clearly see that carbon 2 has 4 different substituents:
(i) a methyl group (CH3)
(ii) a bromine atom (Br)
(iii) a hydrogen atom (H)
(iv) an ethyl group (CH2CH3)
Carbon 2 is a chiral centre and the molecule is chiral.














