Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
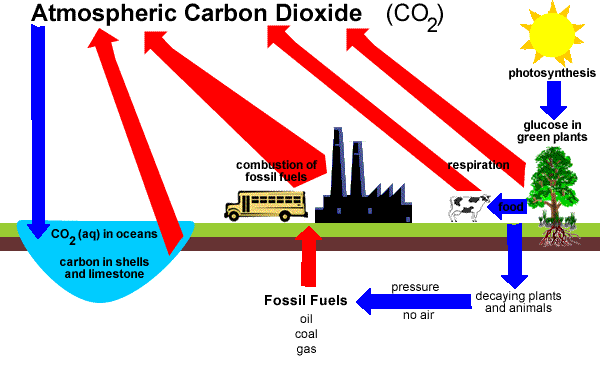
The Carbon Cycle
Green plants convert atmospheric carbon dioxide and water into glucose and oxygen in a process called photosynthesis.
6CO2(g) + 6H2O(l) → C6H12O6(aq) + 6O2(g) ΔH = +2803 kJ mol-1
Photosynthesis is an endothermic reaction. Solar energy from the sun provides the necessary energy for the reaction to proceed.
Animals eat plants, or eat other animals that have eaten plants, and incorporate the carbon atoms into their cells.
Plants and animals both respire.
During respiration, glucose and oxygen are converted into carbon dioxide and water.
C6H12O6(aq) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔH = -2803 kJ mol-1
Respiration is an exothermic reaction, releasing 2803 kJ of energy per mole of glucose consumed in the reaction.
The process of decomposition also releases carbon dioxide back into the atmosphere.
Dead organisms under particular conditions, such as under pressure and in the absence of air, may, over time, be converted into fossil fuels such as coal, oil and gas.
Humans combust these fossil fuels as an energy source which releases carbon dioxide back into the atmosphere.
The complete combustion of coal results in the formation of carbon dioxide gas:
C(s) + O2(g) → CO2(g)
The complete combustion of hydrocarbons, such as methane (CH4(g)), produces carbon dioxide and water:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
The combustion of fossil fuels are exothermic reactions, they release energy.
The total number of carbon atoms is always constant by the Law of Mass Conservation, but there is growing concern over the number of carbon atoms that exist as carbon dioxide because carbon dioxide is a greenhouse gas and a main contributor to the greenhouse effect.