Cohesion
Every particle making up a liquid is attracted to the other particles that surround it in the liquid.
The forces of attraction between molecules making up a liquid are known as intermolecular forces.
On the right, molecules in the liquid are represented as O and the attractive intermolecular forces between the particles in the liquid are shown as red arrows.
Because all the molecules in the liquid are the same, these attractive intermolecular forces of attraction are referred to as cohesive forces.
The particles in the liquid are said to cohere (literally "stick together").
The property of the liquid is known as cohesion.
Every molecule in a liquid is attracted to other molecules in a liquid by the weakest intermolecular forces, known as dispersion forces or London forces.
If a molecule is non-polar, only these weak London forces or dispersion forces attract one molecule to another in the liquid.
If a molecule is polar, then each molecule in the liquid will also experience another, stronger force of attraction.
|
| | ↓ | | ↓ | | ↓ | |
| → | O | →
← | O | →
← | O | ← |
| | ↓ ↑ | | ↓ ↑ | | ↓ ↑ | |
|
|
|
|
|
|
These stronger intermolecular forces of attraction will be either:
- hydrogen bonds
(for polar molecules in which H is covalently bonded to F, O or N in the molecule)
- dipole-dipole interactions
(any other polar molecule)
The stronger the intermolecular forces of attraction acting between the particles in the liquid, the stronger the cohesive force holding the particles together.
| Intermolecular Force |
Relative Strength |
Examples |
|---|
| Dispersion (London) Forces |
weakest |
hexane, benzene, paraffin wax |
| |
↓ |
|
| Dipole-dipole Interactions |
↓ |
hydrogen bromide, chloroform (trichloromethane) |
| |
↓ |
|
| Hydrogen Bonds |
strongest |
water, ethanol |
Adhesion
Adhesion refers to the intermolecular forces attracting a molecule in the liquid phase to a molecule in the solid phase.
|
In the representation on the right:
- molecules in the liquid are represented as O
- attractive intermolecular forces between the particles in the liquid are shown as red arrows
- Cohesive forces are therefore shown as red arrows
- molecules in the solid are represented as O
- attractive intermolecular forces between the particles in the liquid and the particles in the solid are shown as green arrows
- Adhesive forces are therefore shown as green arrows
|
| Liquid Phase |
| | ↓ | | ↓ | | ↓ | |
| → | O | →
← | O | →
← | O | ← |
| | ↓ ↑ | | ↓ ↑ | | ↓ ↑ | cohesion |
|
| → | O | →
← | O | →
← | O | ← |
| | ↑ ↓ | | ↑ ↓ | | ↑ ↓ | adhesion |
|
| O | O | O | O | O | O | O | O | O | O | O | O | O | O |
| Solid Phase |
|
|
The strength of the adhesive force (adhesion) between molecules in the liquid and molecules in the solid will therefore depend on the nature of the molecules making up the liquid and the solid.
The more similar the molecules in the liquid and the molecules in the solid are, the stronger the adhesive force is likely to be.
For example, water molecules are polar and the surface of silica glass is polar so the force of adhesion between water and silica is likely to be quite strong.
Water is polar but paraffin wax is non-polar, so the force of adhesion between water and wax is likely to be quite weak.
Hexane is non-polar and paraffin wax is non-polar, so the force of adhesion between hexane and wax is likely to be quite strong.
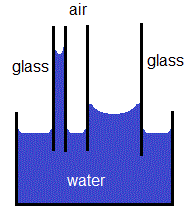
Shape of a Meniscus
Water can wet the surface of glass because the adhesive force of attraction between water molecules and the glass surface is greater than the cohesive forces of attraction between the water molecules.
If a silica glass tube is positioned vertically in a reservoir of water, the same forces of adhesion and cohesion are at work.
In the representation below, we are looking at a cross-section of water molecules inside a silica glass capillary tube:
| |
|
|
|
|
|
adhesion |
|
|
cohesion |
|
|
adhesion |
|
|
|
|
|
|
| s |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
s |
| i |
|
O |
|
|
|
|
|
|
|
Hδ+ |
..... |
water molecules |
..... |
δ+H |
|
|
|
|
|
|
|
O |
|
i |
| l |
|
| |
|
|
|
|
|
|
/ |
|
|
|
|
|
\ |
|
|
|
|
|
|
| |
|
l |
| i |
- |
Si |
- |
Oδ- |
- |
Hδ+ |
...... |
δ-O |
|
|
|
|
|
|
|
Oδ- |
...... |
δ+H |
- |
δ-O |
- |
Si |
- |
i |
| c |
|
| |
|
|
|
|
|
|
\ |
|
|
|
|
|
/ |
|
|
|
|
|
|
| |
|
c |
| a |
|
O |
|
|
|
|
|
|
|
Hδ+ |
..... |
water molecules |
..... |
δ+H |
|
|
|
|
|
|
|
O |
|
a |
| |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| |
|
Oδ- of water attracted
to Hδ+ of silica glass |
|
Oδ- of one water attracted
to Hδ+ of another water |
|
Oδ- of water attracted
to Hδ+ of silica glass |
|
|
Because the forces of adhesion are greater than the forces of cohesion, the water molecules prefer to make hydrogen bonds with the silica glass rather than with other water molecules.
 This causes the water molecules close to the silica glass to creep up the glass, wetting more of the glass.
This causes the water molecules close to the silica glass to creep up the glass, wetting more of the glass.
At the same time, the forces of attraction between water molecules at the boundary between water and air are unbalanced, so the water molecules at this surface are experiencing a pull towards the body of the liquid water (surface tension).
The result of both of these forces, cohesion and adhesion, is the formation of the familiar concave mensicus shape for water in a capillary tube.
Compared to water molecules in liquid water, mercury has much stronger intermolecular forces of attraction acting between the mercury atoms, metallic bonds, so the cohesive force for mercury is much stronger than the cohesive force for water.
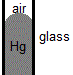 If mercury is placed in a silica glass capillary tube, the force of cohesion between mercury atoms is greater than the force of adhesion between mercury atoms and the silica glass.
If mercury is placed in a silica glass capillary tube, the force of cohesion between mercury atoms is greater than the force of adhesion between mercury atoms and the silica glass.
Mercury atoms therefore "pull away" from the silica glass surface, wetting less of the glass, while the cohesive forces of attraction between the mercury atoms at the boundary between mercury and air are unbalanced and pull the mercury atoms towards the body of the liquid mercury.
This results in the formation of the familiar convex mensicus for mercury in a glass capillary tube.
To summarise:
- cohesion > adhesion → convex meniscus
- cohesion < adhesion → concave meniscus
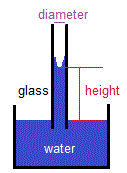
 The shape of the meniscus has important implications when measuring the volume of liquids.
The shape of the meniscus has important implications when measuring the volume of liquids.
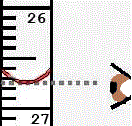
You will have been taught to always measure the volume of liquid in a measuring cylinder, burette, pipette, etc, by reading from the bottom of the meniscus when viewed at eye level.
Why?
Because the glassware you use has been calibrated to measure volumes of water, taking into account the shape, and hence the volume, of the meniscus.
This means that if you measure the volume of water in the apparatus above the bottom of the meniscus, you will be overstating the volume of water.
If you measure the volume of water in the apparatus below the bottom of the meniscus, you will be understating the volume of water.
What happens if you use your apparatus to measure the volume of a liquid other than water?
The truth is that your measurement will only be approximate, because the shape of the meniscus, and hence its volume, will be different to that of water.
You could re-calibrate the apparatus specifically to measure volumes of a different liquid if you need a more accurate measurement.

 This causes the water molecules close to the silica glass to creep up the glass, wetting more of the glass.
This causes the water molecules close to the silica glass to creep up the glass, wetting more of the glass.
 If mercury is placed in a silica glass capillary tube, the force of cohesion between mercury atoms is greater than the force of adhesion between mercury atoms and the silica glass.
If mercury is placed in a silica glass capillary tube, the force of cohesion between mercury atoms is greater than the force of adhesion between mercury atoms and the silica glass.
 The shape of the meniscus has important implications when measuring the volume of liquids.
The shape of the meniscus has important implications when measuring the volume of liquids.