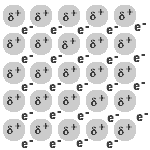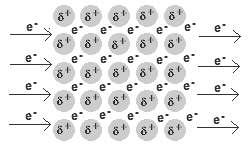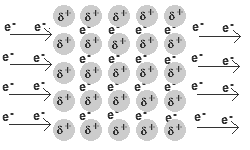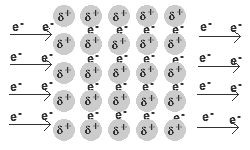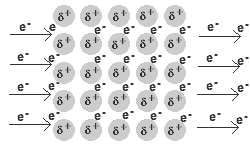Metallic Bonding and the Physical Properties of Metals Chemistry Tutorial
Key Concepts
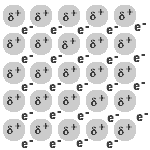
- A metal is a lattice of positive metal 'ions' in a 'sea' of delocalised electrons.
- Metallic bonding refers to the interaction between the delocalised electrons and the metal nuclei.
- The physical properties of metals are the result of the delocalisation of the electrons involved in metallic bonding.
- The physical properties of solid metals are:
⚛ conduct heat
⚛ conduct electricity
⚛ generally high melting points and high boiling points
⚛ strong
⚛ malleable (can be hammered or pressed out of shape without breaking)
⚛ ductile (able to be drawn into a wire)
⚛ metallic lustre
⚛ opaque (reflect light)
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Physical Properties of Metals: Conductivity of Heat and Electricity
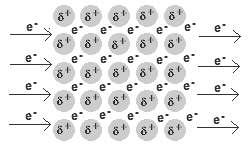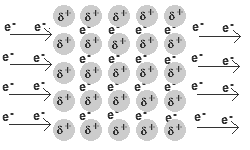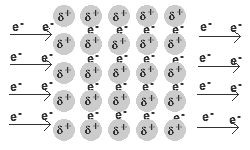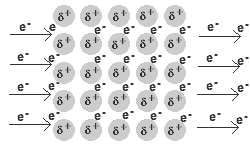
Solid and liquid metals conduct heat and electricity.
The delocalised electrons are free to move in the solid lattice.
These mobile electrons can act as charge carriers in the conduction of electricity or as energy conductors in the conduction of heat.
The table below shows the electrical conductivity (106Ohm-1cm-1) of the metals lithium to indium.
Group 1
(Alkali Metals) |
Group 2
(Alkali Earth metals) |
Transition Metals
(Groups 3 to 12) |
Group 13 |
|---|
Li
0.108 |
Be
0.313 |
|
|
Na
0.210 |
Mg
0.226 |
|
Al
0.377 |
K
0.139 |
Ca
0.298 |
Sc
0.017 |
Ti
0.023 |
V
0.048 |
Cr
0.077 |
Mn
0.006 |
Fe
0.093 |
Co
0.172 |
Ni
0.143 |
Cu
0.596 |
Zn
0.166 |
Ga
0.067 |
Rb
0.077 |
Sr
0.076 |
Y
0.016 |
Zr
0.023 |
Nb
0.069 |
Mo
0.187 |
Tc
0.067 |
Ru
0.137 |
Rh
0.211 |
Pd
0.095 |
Ag
0.630 |
Cd
0.138 |
In
0.116 |
Physical Properties of Metals: High Melting Point
In general, metals have high melting and boiling points because of the strength of the metallic bond.
The strength of the metallic bond depends on the:
- number of electrons in the delocalised 'sea' of electrons.
(More delocalised electrons results in a stronger bond and a higher melting point.)
- packing arrangement of the metal atoms.
(The more closely packed the atoms are the stronger the bond is and the higher the melting point.)
The table below shows the approximate melting points (°C) of the metals from lithium to indium.
Group 1
(Alkali Metals) |
Group 2
(Alkali Earth metals) |
Transition Metals
(Groups 3 to 12) |
Group 13 |
|---|
Li
180.7 |
Be
1,278 |
|
|
Na
98 |
Mg
650 |
|
Al
660 |
K
63.35 |
Ca
839 |
Sc
1,539 |
Ti
1,660 |
V
1,902 |
Cr
1,857 |
Mn
1,246 |
Fe
1,535 |
Co
1,495 |
Ni
1,453 |
Cu
1,085 |
Zn
419.73 |
Ga
30 |
Rb
39.64 |
Sr
769 |
Y
1,526 |
Zr
1,852 |
Nb
2,468 |
Mo
2,617 |
Tc
2,200 |
Ru
2,250 |
Rh
1,966 |
Pd
1,552 |
Ag
961 |
Cd
321 |
In
157 |
Group 1 (alkali) metals have relatively low melting points compared to other metals because they:
- only have 1 electron to contribute to the delocalised 'sea' of electrons
- are not forming as many metallic bonds as other metals because Group 1 atoms are inefficiently packed
- have large atomic radii so the delocalised electrons are further away from the nucleus resulting in a weaker metallic bond
Physical Properties of Metals: Malleability and Ductility
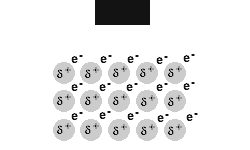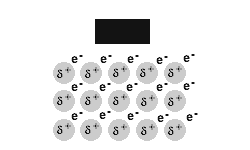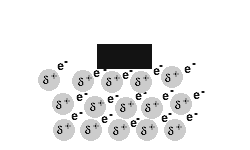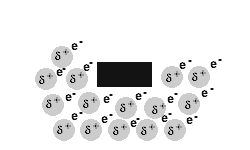
Metals are malleable and ductile.
The delocalised electrons in the 'sea' of electrons in the metallic bond, enable the metal atoms to roll over each other when a stress is applied.
Physical Properties of Metals: Optical Properties
Metals typically have a shiny, metallic lustre.
Photons of light do not penetrate very far into the surface of a metal and are typically reflected, or bounced off, the metallic surface.