Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Equilibrium Considerations
The reaction between nitrogen gas and hydrogen gas to produce ammonia gas is an exothermic equilibrium reaction, releasing 92.4 kJ mol-1 of energy at 298 K (25oC).
N2(g)
nitrogen |
+ |
3H2(g)
hydrogen |
heat, pressure, catalyst
 |
2NH3(g)
ammonia |
ΔH = -92.4 kJ mol-1 |
OR, including the enthalpy change as a product of the reaction:
N2(g)
nitrogen |
+ |
3H2(g)
hydrogen |
heat, pressure, catalyst
 |
2NH3(g)
ammonia |
+ 92.4 kJ mol-1 |
By Le Chetalier's Principle, increasing the pressure on the reaction mixture favours the formation of ammonia gas:
Increasing the pressure causes the equilibrium position to move to the right resulting in a higher yeild of ammonia since there are more gas molecules on the left hand side of the equation (4 in total) than there are on the right hand side of the equation (2).
Increasing the pressure means the system adjusts to reduce the effect of the change, that is, to reduce the pressure by having fewer gas molecules.
By Le Chetalier's Principle, decreasing the temperature of the reaction mixture favours the formation of ammonia gas:
Decreasing the temperature causes the equilibrium position to move to the right resulting in a higher yield of ammonia since the reaction is exothermic (releases heat).
Reducing the temperature means the system will adjust to minimise the effect of the change, that is, it will produce more heat since energy is a product of the reaction, and will therefore produce more ammonia gas as well.
However, the rate of the reaction at lower temperatures is extremely slow, so a higher temperature must be used to speed up the reaction which results in a lower yield of ammonia.
The equilibrium expression for this reaction is:
| Kc = |
[NH3(g)]2
[N2(g)][H2(g)]3
|
As the temperature increases, the value of the equilibrium constant decreases as the yield of ammonia decreases.
Temperature
(oC) |
Kc |
trend |
|---|
| 25 |
6.4 x 102 |
higher Kc |
|---|
| 200 |
4.4 x 10-1 |
↓ |
|---|
| 300 |
4.3 x 10-3 |
↓ |
|---|
| 400 |
1.6 x 10-4 |
↓ |
|---|
| 500 |
1.5 x 10-5 |
lower Kc |
|---|
In summary, higher pressure favours formation of ammonia gas, lower temperature favours formation of ammonia gas BUT the reaction rate is slow at lower temperatures.
Reaction Rate (Kinetic) Considerations
- A catalyst such as an iron catalyst is used to speed up the reaction by lowering the activation energy so that the N2 bonds and H2 bonds can be more readily broken.
- Increased temperature means more reactant molecules have sufficient energy to overcome the energy barrier to reacting (activation energy) so the reaction is faster at higher temperatures (but the yield of ammonia is lower as discussed above).
A temperature range of 400-500oC is a compromise designed to achieve an acceptable yield of ammonia (10-20%) within an acceptable time period.
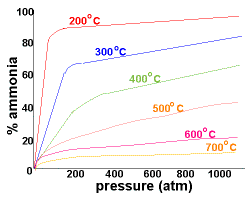
| Refer to the graph on the right which records the yield of ammonia as a percentage at different pressures:
At 200oC and pressures above 750 atm there is an almost 100% conversion of reactants to the ammonia product.
Since there are difficulties associated with containing larger amounts of materials at this high pressure, lower pressures of around 200 atm are used industrially.
By using a pressure of around 200 atm and a temperature of about 500oC, the yield of ammonia is 10-20%, while costs and safety concerns in the building and during operation of the plant are minimised
|
 |
During industrial production of ammonia, the reaction never reaches equilibrium as the gas mixture leaving the reactor is cooled to liquefy and remove the ammonia.
The remaining mixture of reactant gases are recycled through the reactor.
The heat released by the reaction is removed and used to heat the incoming gas mixture.
A Brief History of Ammonia Production
At the beginning of the 20th century there was a shortage of naturally occurring, nitrogen-rich fertilisers, such as Chile saltpetre, which prompted the German Chemist Fritz Haber, and others, to look for ways of combining the nitrogen in the air with hydrogen to form ammonia, which is a convenient starting point in the manufacture of fertilisers.
This process was also of interest to the German chemical industry as Germany was preparing for World War I and nitrogen compounds were needed for explosives.
The hydrogen for the ammonia synthesis was made by the water-gas process (a Carl Bosch invention) which involves blowing steam through a bed of red hot coke resulting in the separation of hydrogen from oxygen.
The nitrogen was obtained by distillation of liquid air, then by cooling and compressing air.
These days, the hydrogen is produced by reforming light petroleum fractions or natural gas (methane, CH4) by adding steam:
| CH4(g) + H2O(g) |
Ni catalyst
→
700oC |
CO(g) + 3H2(g) |
Enough steam is used to react with about 45% of the methane (CH4), the rest of the methane is reacted with air:
| 2CH4(g) + | O2(g) + 4N2(g)
(air) |
Ni catalyst
→
| 2CO(g) + 4H2(g) + 4N2(g) |
All the carbon monoxide (CO) in the mixture is oxidised to CO2 using steam and an iron oxide catalyst:
| CO(g) + H2O(g) |
iron oxide catalyst
→
|
H2(g) + CO2(g) |
The carbon dioxide (CO2) is removed using a suitable base so that only the nitrogen gas (N2) and hydrogen gas (H2) remain and are used in the production of ammonia (NH3).
In ammonia production the hydrogen and nitrogen are mixed together in a ratio of 3:1 by volume and compressed to around 200 times atmospheric pressure.


