Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Gas Chromatography Techniques
Step 1:
Sample Injection |
Step 2:
Separation in Column |
Step 3:
Detection & Recording |
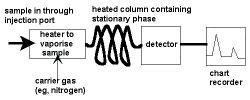 |
Step 1: Sample Injection
A small amount of liquid sample to be analysed is drawn up into a syringe.
Typical volumes used are between 0.1 μL and 1 mL.
The syringe needle is positioned in the hot injection port of the gas chromatograph and the sample is injected quickly.
The injection of the sample is considered to be a "point" in time, that is, it is assumed that the entire sample enters the gas chromatograph at the same time, so the sample must be injected quickly. This also explains why it is beneficial to use very small amounts of sample.
The temperature is set to be higher than the boiling points of the components of the mixture so that the components will vaporise.
The vaporised components then mix with the inert gas mobile phase to be carried to the gas chromatography column to be separated.
This mobile phase carrier gas must not react with any of the components of the mixture, if it did react we would not be able to identify the components in the mixture! For the same reason, it is essential that the components we are analysing for do not decompose into other substances when heated.
Step 2: Separation in the Column
Components in the mixture are separated based on their abilities to adsorb on, or bind to, the stationary phase.
A component that adsorbs most strongly to the stationary phase will spend the most time in the column (will be retained in the column for the longest time) and will therefore have the longest retention time (Rt).
It will emerge from the gas chromatograph last.
A component that adsorbs the least strongly to the stationary phase will spend the least time in the column (will be retained in the column for the shortest time) and will therefore have the shortest retention time (Rt).
It will emerge from the gas chromatograph first.
One of the most important factors in determining how strongly a molecule will adsord on, or bind to, the stationary phase is the relative polarity of the component molecule and the stationary phase.
If we consider a 2 component mixture in which component A is more polar than component B then:
- component A will have a longer retention time in a polar column than component B
- component A will have a shorter retention time in a non-polar column than component B
| Polarity of Components in a Mixture |
|---|
| polar component | non-polar component |
|---|
c
o
n
d
i
t
i
o
n
s | polar stationary phase | Adsorbs strongly to stationary phase.
Retained in column for long time.
Large Rt | Adsorbs weakly to stationary phase.
Retained in column for short time.
Small Rt |
|---|
| non-polar stationary phase | Adsorbs weakly to stationary phase.
Retained in column a short time.
Small Rt | Adsorbs strongly to stationary phase.
Retained in column a long time.
Large Rt |
|---|
The temperature of the column affects the speed with which the vaporised substances pass through the column.
The hotter the column, the faster the gases travel through it.
Step 3: Detecting and Recording Results
The components of the mixture reach the detector at different times due to differences in the time they are retained in the column.
The component that is retained the shortest time in the column is detected first.
The component that is retained the longest time in the column is detected last.
The detector sends a signal to the chart recorder which results in a peak on the chart paper.
The component that is detected first is recorded first.
The component that is detected last is recorded last.
The gas chromatogram (chromatograph) below shows 2 peaks due to different substances in the mixture.
Substance A was the first to emerge from the column, the first to be detected and the first to be recorded.
Substance A was retained the shortest period of time in the column.
Substance A has the shortest retention time, Rt.
Substance A is less strongly attracted to the stationary phase.
|
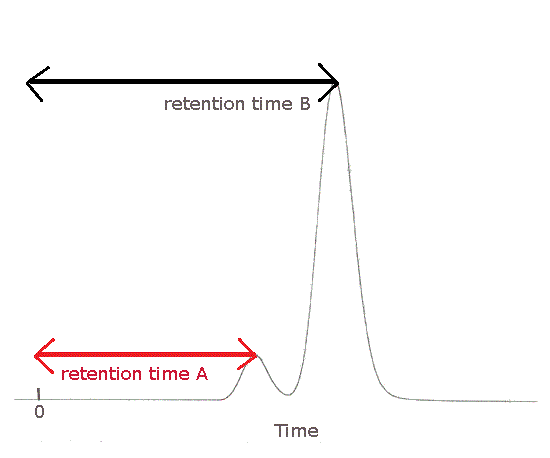 |
Substance B was the last to emerge from the column, the last to be detected and the last to be recorded.
Substance B was retained the longest period of time in the column.
Substance B has the longest retention time, Rt.
Substance B is most strongly attracted to the stationary phase.
|
| The area of the peak for Substance A has been shaded in red.
Substance A's peak area is less than Substance B's peak area.
The sample contained less Substance A than Substance B.
|
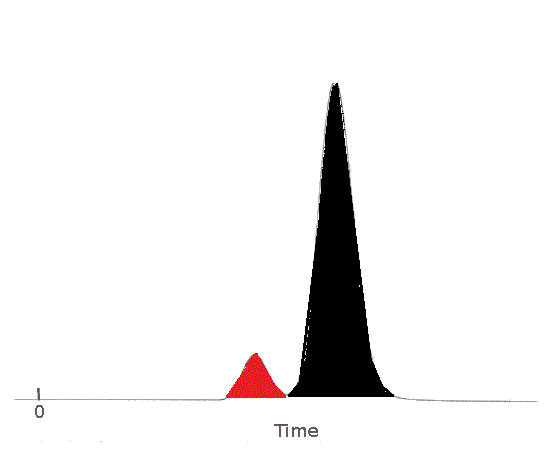 |
The area of the peak for Substance B has been shaded in black.
Substance B's peak area is greater than Substance A's peak area.
The sample contained more Substance B than Substance A.
|
1. Comparing peak areas to determine relative concentration of component substances in a mixture assumes that each component behaves in a similar manner in the column.



