Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Preparation of Ethanol by Fermentation of Glucose in the Laboratory
Producing ethanol of a high concentration is a two-step process:
- Fermentation of glucose to produce the ethanol (ethyl alcohol)
- Distillation of the fermentation products to purify the ethanol (ethyl alcohol)
Step 1: Fermentation of Glucose to Produce Ethanol
The procedure used to ferment glucose in the school laboratory is set out below:
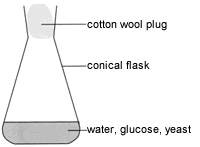
- Glucose is dissolved in warm water in a conical flask.
- Some yeast is added to the glucose solution and cotton wool is used to plug the top of the conical flask.
- The flask is left in a warm place to allow fermentation to take place.
- When bubbles of carbon dioxide are no longer forming, the fermentation reaction has ceased.
The chemistry of the fermentation of glucose to produce ethanol is quite complicated.
A simplified outline is given below.
The first step in the fermentation reaction is the conversion of glucose (C6H12O6) to pyruvic acid (CH3-CO-COOH) using the hydrogen carrier NADH present in the yeast (1):
C6H12O6 + 2NAD+ → 2CH3-CO-COOH + 2NADH + 2H+
NAD+ has been reduced to NADH, it has gained hydrogen.(2)
Glucose has been oxidised, oxygen has been gained to produce pyruvic acid.
Zymase, an enzyme in the yeast, converts the pyruvic acid to ethanol and carbon dioxide using the hydrogen carrier NADH:
CH3-CO-COOH + NADH + H+ → C2H5OH + CO2 + NAD+
NADH has been oxidised to NAD+ through the loss of hydrogen.
Pyruvic acid has lost oxygen to produce ethanol so it has been reduced.
So, the overall equation for the fermentation of glucose is:
| C6H12O6 |
+ |
2NAD+ |
|
|
→ |
2CH3-CO-COOH |
+ |
2NADH |
+ |
2H+ |
2CH3-CO-COOH |
+ |
2NADH |
+ |
2H+ |
→ |
2C2H5OH |
+ |
2CO2 |
+ |
2NAD+ |
|
| C6H12O6 |
|
|
|
|
→ |
2C2H5OH |
+ |
2CO2 |
|
|
In reality, the chemistry of fermentation is actually much more complex than this, with the glucose being incorporated into other by-products such as pyruvic acid, acetaldehyde and glycerol.
Distillation of Fermentation Products to Produce Ethanol
Distillation is a technique that separates a mixture of different compounds with different boiling points.
At a pressure of 1 atmosphere (or 101.3kPa) the boiling point of pure water is 100°C while the boiling point of ethanol is 78.3°C.
Because ethanol and water have different boiling points we can separate a mixture of ethanol and water by boiling it.
Ethanol, with the lower boiling point, will boil first. We can condense the hot vapor using a water-cooled condenser so that the liquid ethanol can be collected in a separate vessel.
Once all the ethanol has boiled off, the temperature of the solution will start increasing again until the water starts to boil off at 100°C.
We stop collecting the liquid as soon as the temperature of the solution starts to increase so that we don't collect water in the same vessel that contains our ethanol!
The procedure for distilling your ethanol/water mixture is set out below:
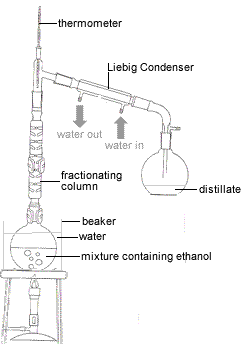
- The mixture containing ethanol is placed in a round-bottomed flask or Claisen flask.
- Since ethanol is flammable and has a boiling point below that of water, the flask is placed in a water bath to gently heat the mixture.
- Using a fractionating column reduces the number of distillations required to complete the separation of the ethanol as it provides a continuous series of partial condensations of the vapour and partial vaporisations of the condensate.
- A thermometer is used to ensure that only the fraction that boils at 78.3°C is collected as this is the ethanol fraction.
- The ethanol vapor entering the Liebig condenser (water-cooled condenser) is cooled and condenses to liquid ethanol.
One end of a flexible tube is connected to the bottom of the Liebig condenser and the other end is connected to a water tap.
One end of a flexible tube is connected the top of the Liebig condenser and the other end of this tube is placed in the sink.
When the tap is turned on, water flows into the bottom of the Liebig condenser and out of the top of the Liebig condenser.
This constant stream of cool water allows heat to be transferred from the hot vapour in the Liebig condenser to the cooler water which is flowing away, thereby condensing the vapour to the liquid.
- The product of the distillation is called the distillate and is collected in a suitable clean, dry flask (often a conical flask also known as an Erlenmeyer flask).
The distillate will contain ethanol as long as the temperature of the vapour as measured by the thermometer is 78.3oC.
When the temperature of the vapour begins to rise, the flask containing the distillate should be removed and replaced with a clean flask to prevent contamination of the ethanol distillate, and the bunsen burner turned off.
In practice, it is not possible to obtain pure 100% ethanol using this distillation method because the constant boiling point mixture collected as the distillate contains about 95% ethanol and 5% water. (3)
Further concentration of the ethanol is possible using other separation techniques such as vacuum distillation, or, by adding another substance such as benzene to the mixture.


